Day 1 :
Keynote Forum
Henry M Sobell
Emeritus Professor
Keynote: The centers of premeltons signal the beginning and ends of genes

Biography:
Henry M. Sobell completed his studies at Brooklyn Technical High School (1948-1952), Columbia College (1952-1956), and the University of Virginia School of Medicine (1956-1960). Instead of practicing clinical medicine, he then went to the Massachusetts Institute of Technology (MIT) to join Professor Alexander Rich in the Department of Biology (1960-1965), where, as a Helen Hay Whitney Postdoctoral Fellow, he learned the technique of single crystal X-ray analysis. He then joined the Chemistry Department at the University of Rochester, having been subsequently jointly appointed to both the Chemistry and Molecular Biophysics departments (the latter at the University of Rochester School of Medicine and Dentistry), becoming a full tenured Professor in both departments (1965-1993). He is now retired and living in the Adirondacks in New York, USA.
Abstract:
Premeltons are examples of emergent structures (i.e., structural solitons) that arise spontaneously in DNA due to the presence of nonlinear excitations in its structure. They are of two kinds: B-B (or A-A) premeltons form at specific DNA-regions to nucleate site-specific DNA melting. These are stationary and, being globally nontopological, undergo breather motions that allow drugs and dyes to intercalate into DNA. B-A (or A-B) premeltons, on the other hand, are mobile, and being globally topological, act as phase-boundaries transforming B- into A- DNA during the structural phase-transition. They are not expected to undergo breather-motions. A key feature of both types of premeltons is the presence of an intermediate structural-form in their central regions (proposed as being a transition-state intermediate in DNA-melting and in the B- to A- transition), which differs from either A- or B- DNA. Called beta-DNA, this is both metastable and hyperflexible – and contains an alternating sugar-puckering pattern along the polymer-backbone combined with the partial-unstacking (in its lower energy-forms) of every other base-pair. Beta-DNA is connected to either B- or to A- DNA on either side by boundaries possessing a gradation of nonlinear structural-change, these being called the kink and the antikink regions. The presence of premeltons in DNA leads to a unifying theory to understand much of DNA physical-chemistry and molecular-biology. In particular, premeltons are predicted to define the 5’ and 3’ ends of genes in naked-DNA and DNA in active-chromatin, this having important implications for understanding physical aspects of the initiation, elongation and termination of RNA-synthesis during transcription. For these and other reasons, the model will be of broader interest to the general audience working in these areas. The model explains a wide variety of data, and carries within it a number of experimental predictions – all readily testable – as will be described in my talk.
References:
- Sobell HM, (2016) Premeltons in DNA. Journal of Structural and Functional Genomics 17:17-31.
- Sobell HM, (2013) Organization of DNA in Chromatin. Rather than bending uniformly along its length, nucleosomal DNA is proposed to consist of multiple segments of B- and A- DNA held together by kinks when forming its left-handed toroidal superhelical structure. Explanatory publications. ISBN 978-0-692-01974-0.
Keynote Forum
Shigeyuki Yokoyama
RIKEN Structural Biology Laboratory
Keynote: Structures and functions of seven-transmembrane helix receptors

Biography:
Shigeyuki Yokoyama obtained his PhD degree from The University of Tokyo in 1981. He was associate professor (1986–1991) and professor (1991–2012) at The University of Tokyo, and now is emeritus professor. He was also appointed director of the Cellular Signaling Laboratory (1993–2004), the Structural Molecular Biology Laboratory (2004-2006), the Protein Research Group (1998–2008), and the Systems and Structural Biology Center (2008–2013). He is distinguished senior scientist and directing the Structural Biology Laboratory at RIKEN. He has published more than 800 papers, and has been serving as editorial board members of Nucleic Acids Research etc.
Abstract:
The adiponectin receptors, AdipoR1 and AdipoR2, are key anti-diabetic molecules. AdipoR1 and AdipoR2 are seven transmembrane helix receptor proteins orienting their N- and C-termini on the intracellular and extracellular sides, respectively, which is opposite to G-protein coupled receptors (GPCRs). We determined the crystal structures of human AdipoR1 and AdipoR2, and found that they represent a novel class of receptor structure. The seven transmembrane helices form a large internal cavity, in which three conserved His residues coordinate a zinc ion. This zinc-coordinated structure indicates that AdipoR1 and AdipoR2 are hydrolytic enzymes. Both AdipoR1 and AdipoR2 assume the closed and open forms. The lipids bound in the closed and open forms were identified, which indicated that the zinc-coordinated structure is for lipid hydrolysis.
We determined the crystal structure of a GPCR, leukotriene B4 (LTB4) receptor BLT1, bound with an antagonist. BLT1 exhibits the canonical seven transmembrane helix structure. The binding mode of the antagonist is characteristic, and is expected to be useful for further drug development.
We applied the cell-free protein synthesis method to production of GPCRs. By adding a mixture of mammalian lipids in the cell-free reaction, GPCRs were synthesized and folded with lipids. This method is useful for large-scale production of high quality GPCR samples for structural and functional studies.
References:
- Muramatsu T, Takemoto C, Kim YT, Wang H, Nishii W, Terada T, Shirouzu M, Yokoyama S (2016) SARS-CoV 3CL protease cleaves its C-terminal autoprocessing site by novel subsite cooperativity. Proc. Natl. Acad. Sci. 113(46):12997-13002.
- Shinoda T, Shinya N, Ito K, Ohsawa N, Terada T, Hirata K et. al. (2016) Structural basis for disruption of claudin assembly in tight junctions by an enterotoxin. Sci. Rep. 6:33632.
- Shinoda T, Shinya N, Ito K, Ishizuka-Katsura Y, Ohsawa N et. al. (2016) Cell-free methods to produce structurally intact mammalian membrane proteins. Sci. Rep. 6.
- Kashiwagi K, Takahashi M, Nishimoto M, Hiyama TB, Higo T et. al. (2016) Crystal structure of eukaryotic translation initiation factor 2B. Nature. 531(7592):122-125.
5. Tanabe H, Fujii Y, Okada-Iwabu M, Iwabu M, Nakamura Y et. al. (2015) Crystal structures of the human adiponectin receptors. Nature. 520(7547): 312-316.
Keynote Forum
Tilman Schirmer
University of Basel, Switzerland
Keynote: Structural biology of c-di-GMP mediated signaling

Biography:
Tilman Schirmer is interested in molecular mechanisms of bacterial signal transduction. He is a trained Crystallographer, but has extended his activities into functional characterization and kinetic modeling to reveal structure - function relationships. He has graduated at the Max-Planck Institute in Martinsried (Germany) and has worked at the LMB Cambridge (UK) on the regulation of phosphofructokinase. He then moved to the Biozentrum Basel (Switzerland) to reveal the structure and translocation mechanism of maltoporin. His current interest lies mainly in the various aspects of c-di-GMP signaling and FIC-mediated AMPylation of target proteins.
Abstract:
In addition to the well-known cyclic nucleotides, cAMP and cGMP, bacteria utilize cyclic di-guanosine monophosphate (c-di-GMP) to control various cellular processes. Hereby, the cellular level of the messenger is set by the antagonistic activities of diguanylate cyclases and specific phosphodiesterases. In a given organism, there are usually multiple variants of the two enzymes, which are tightly regulated by a variety of external and internal cues due to the presence of specialized sensory or regulatory domains. Fundamental cellular processes, such as bacterial life style, biofilm formation, and cell cycle control are thus getting controlled in a coordinated fashion by downstream c-di-GMP receptors in response to the input signals.
Crystal structures in combination with biochemical and biophysical analyses reveal that both GGDEF diguanylate cyclase and EAL phosphodiesterase domains are active only as homotypic dimers. In the full-length enzymes, attainment of the competent quarternary structure depends on the signaling state of the accessory domains (e.g. Rec, PAS, GAF), that typically dimerize or change their dimeric structure upon signal perception. Histidine kinases and transcription factors use very similar regulatory domains to control output function in a dimeric context. It can be inferred that the modular arrangement of catalytic and regulatory dimers, both forming homotypic interactions, facilitates their recombination during evolution.
As an example for c-di-GMP mediated allosteric control of a downstream effector, the effect of c-di-GMP binding to the bifunctional histidine kinase CckA from C. crescentus will be presented. It was found that c-di-GMP promotes the phosphatase activity of the enzyme via stabilization of the phosphatase competent constellation due to non-covalent domain cross-linking. In silico analyses predict that c-di-GMP control is widespread among bacterial histidine kinases, arguing that it can replace or modulate canonical transmembrane signaling.
References:
- Schirmer, T (2016) C-di-GMP Synthesis: Structural Aspects of Evolution, Catalysis and Regulation. J. Mol. Biol. 428(19):3683–3701.
- Dubey B, Lori C, Ozaki S, Fucile G, Plaza Menacho I, Jenal U, and Schirmer T (2016) Cyclic di-GMP mediates a histidine kinase/phosphatase switch by noncovalent domain cross-linking. Science Advances. 2(9):e1600823.
- Reinders A, Hee C S, Ozaki S, Mazur A, Boehm A, Schirmer T, Jenal U (2016) Expression and Genetic Activation of Cyclic di-GMP-specific phosphodiesterases in escherichia coli. Journal of Bacteriology. 198(3):448–462.
- Sundriyal A, Massa C, Samoray D, Zehender F, Sharpe T, Jenal U, Schirmer T (2014) Inherent regulation of EAL domain-catalyzed hydrolysis of second messenger cyclic di-GMP. J. Biol. Chem. 289(10):6978–6990.
- Schirmer T, Jenal U (2009) Structural and mechanistic determinants of c-di-GMP signalling. Nat. Rev. Microbiol. 7(10):724–735.
- Special Session: Structural Biology & Single Molecules
Chair
Yuri L Lyubchenko
University of Nebraska Medical Center, USA
Session Introduction
Yuri L. Lyubchenko
University of Nebraska Medical Center, USA
Title: Nanoscale structure and dynamics of centromere nucleosomes
Time : 10:50-11:10

Biography:
Yuri L. Lyubchenko is Professor of Pharmaceutical Sciences at the University of Nebraska Medical Center, Omaha, NE, USA. His research focuses on understanding fundamental mechanisms underlying health and disease, which are key to developing new and more effective diagnostics and medications. This primarily basic research allows him not only identify new drug targets for small molecule drugs, it also leads to development of the nanotools and methods to discover novel approaches for diagnostic, treatment and disease prevention and to more rapidly determine their efficacy at the molecular level.
Abstract:
Statement of the Problem: Chromatin integrity is crucial for normal cell development. The cell division process is accompanied by the segregation of replicated chromosome, and chromatin centromeres, specialized segments of chromosomes provide the accuracy of the chromosomal segregation. If the centromere becomes damaged or removed, chromosomes segregate randomly disrupting the cell division process. The centromeres are specifically recognized by kinetochores suggesting that centromeres contain specific structural characteristics. However, these structural details and the mechanism underlying their highly specific recognition remain uncertain. Methodology & Theoretical Orientation: In this study, we performed direct imaging of CENP-A nucleosome core particles by time-lapse high-speed atomic force microscopy (AFM), enabling us to directly visualize the dynamics of CENP-A nucleosomes. Nucleosomes used for evaluation of DNA wrapping around the histone core were assembled on a DNA substrate containing a centrally positioned 601 motif. Findings: A broadly dynamic behavior of the DNA flanks was first revealed by analysis of AFM images acquired in ambient conditions. Time-lapse imaging further identified the distinctive pathways unique to CENP-A-nucleosome dynamics that are not shared by H3. The spontaneous unwrapping of DNA flanks can be accompanied by the reversible and dynamic formation of loops with sizes equivalent to a single wrap of DNA. Translocation of CENP-A nucleosomes was observed, with the formation of internal DNA loops along the nucleosome. This process was reversible, settling the core back to its starting position. Additionally, the transfer of the histone core from one DNA substrate to another was visualized, as well as distinctive splitting into sub-nucleosomal particles that was also reversible. Conclusion & Significance: Altogether, our data suggest that unlike H3, CENP-A is very dynamic, permitting its nucleosome to distort freely and reversibly, which in turn allows a longer term stability, which may play a critical role in centromere integrity during mitosis and replication.
References:
[1] Lyubchenko, Y. L. (2014) Centromere chromatin: a loose grip on the nucleosome?, Nat Struct Mol Biol 21, 8.
[2] Lyubchenko, Y. L., and Shlyakhtenko, L. S. (2015) Chromatin imaging with time-lapse atomic force microscopy, Methods Mol Biol 1288, 27-42.
[3] Lyubchenko, Y. L., and Shlyakhtenko, L. S. (2015) Atomic Force Microscopy Imaging and Probing of Amyloid Nanoaggregates, In Handbook of Clinical Nanomedicine: From Bench to Bedside (Bawa, R., Audette, G. & Rubinstein, I., Ed.), p 1500. , Pan Stanford Publishing, Singapore. .
[4] Sun, Z., Tan, H. Y., Bianco, P. R., and Lyubchenko, Y. L. (2015) Remodeling of RecG Helicase at the DNA Replication Fork by SSB Protein, Sci Rep 5, 9625.
[5] Proctor, E. A., Fee, L., Tao, Y., Redler, R. L., Fay, J. M., Zhang, Y., Lv, Z., Mercer, I. P., Deshmukh, M., Lyubchenko, Y. L., and Dokholyan, N. V. (2016) Nonnative SOD1 trimer is toxic to motor neurons in a model of amyotrophic lateral sclerosis, Proc Natl Acad Sci U S A 113, 614-619.
John van Noort
Leiden University, The Netherlands
Title: The structure of chromatin; single-molecule experiments on model fibers and real genes

Biography:
Chromatin is the ubiquitous protein-DNA complex that forms the structural basis of DNA condensation in all eukaryotic organisms. Packaging and depackaging of chromatin, called chromatin remodeling, plays a central role in all cellular processes that involve chromosomes such as transcription, replication, recombination and repair. Detailed knowledge of the principles and mechanisms underlying this control of DNA condensation is thus vital for understanding many diseases, including neurological disorders and cancer. The physical mechanisms governing these processes however, are still largely unknown. I am interested in developing and using modern biophysical techniques to unravel the physics behind DNA condensation and its role in transcription regulation.
Abstract:
The folding of chromatin defines access to our genes and therefore plays a pivotal role in transcription regulation. However, the structure of chromatin fibers is poorly defined and heavily debated. We used single-molecule techniques to probe and manipulate the dynamics of nucleosomes in individual chromatin fibers. These novel methods were initially applied to synthetic, highly homogeneous nucleosomal arrays and yielded unprecedented insight in the structure and dynamics of chromatin.
With single pair Forster Resonance Energy Transfer we showed that the nucleosome is very dynamic, unwrapping half of its DNA four times per second. Using single molecule force spectroscopy, it was possible to measure the kinetics of this unfolding, both in single nucleosomes and in well-defined arrays of nucleosomes that fold into a 30 nm fiber. Analysis of the unfolding pattern reveals a linker length dependence of the higher order folding.
The linker length in vivo however varies, and to obtain insight the positioning of nucleosomes we developed a simple statistical physics model that captures sequence dependent positioning effects for both reconstitutions on synthetic DNA and chromatin in vivo.
We recently developed a method to purify specific chromatin fragments from yeast without crosslinking the fiber while maintaining the complexity that provides functionality to our epi-genome. I will show the first single-molecule force spectroscopy results on intact, native fibers which uniquely probe chromatin structure, composition and variations in it at the single-molecule level.
Refernces:
-
Multiplexing genetic and nucleosome positioning codes: a computational approach (2016) B Eslami-Mossallam, RD Schram, M Tompitak, J van Noort, H Schiessel PloS one 11 (6), e0156905
-
Quantitative analysis of single-molecule force spectroscopy on folded chromatin fibers (2016) H Meng, K Andresen, J Van Noort Nucleic acids research 43 (7), 3578-3590
-
spFRET reveals changes in nucleosome breathing by neighboring nucleosomes (2015) R Buning, W Kropff, K Martens, J van Noort Journal of Physics: Condensed Matter 27 (6), 064103
-
Histone H3 phosphorylation near the nucleosome dyad alters chromatin structure (2014) Justin A North, Marek Šimon, Michelle B Ferdinand, Matthew A Shoffner, Jonathan W Picking, Cecil J Howard, Alex M Mooney, John van Noort, Michael G Poirier, Jennifer J Ottesen Nucleic acids research 42 (8), 4922-4933
-
Sequence-based prediction of single nucleosome positioning and genome-wide nucleosome occupancy (2012) T van der Heijden, JJFA van Vugt, C Logie, J van Noort Proceedings of the National Academy of Sciences 109 (38), E2514-E25225.
Peter Hinterdorfer
Johannes Kepler University Linz , Austria
Title: Deciphering and filming molecular recognition at the nano-scale with AFM
Time :

Biography:
Peter Hinterdorfer performs advanced nanoscopic techniques in nano-bio technology, life science, and medical diagnostics, and has been working on antibody/antigen interactions, transmembrane transporters, virus/membrane interactions, cells of the immune system, nuclear envelope membranes, and bacterial surface layers. He has done pioneering work in single molecule force spectroscopy and has invented a combined topography and recognition imaging technique. Recently he did research with high-speed bio-AFM.
Abstract:
In molecular recognition force microscopy (MRFM), ligands are covalently attached to atomic force microscopy tips for the molecular recognition of their cognitive receptors on probe surfaces1. Interaction forces between single receptor-ligand pairs are measured in force-distance cycles. The dynamics of the experiment is varied2, which gives insight into the molecular dynamics of the receptor-ligand recognition process and yields information about the binding pocket, binding energy barriers, and kinetic reaction rates3. Combination of high-resolution atomic force microscope topography imaging with single molecule force spectroscopy provides a unique possibility for the localization of specific molecular recognition events4. The identification and visualization of receptor binding sites on complex heterogeneous bio-surfaces such as cells and membranes are of particular interest in this context4. Considered as the paradigm for molecular recognition are antibodies. They are key molecules for the immune system of vertebrates. The Y-shaped antibody type IgG exhibits C2-symmetry; its Fc stem is connected to two identical Fab arms, binding antigens by acting as molecular callipers. Bivalent binding of the two Fab arms to adjacent antigens can only occur within a distance of roughly 6 to 12 nm. AFM cantilevers adorned with an antibody can measure the distances between 5-methylcytidine bases in individual DNA strands with a resolution of 4Å, thereby revealing the DNA methylation pattern6, which has an important role in the epigenetic control of gene expression. Moreover, due to their nano-mechanical properties antibodies exhibit “bipedal” walking on antigenic surfaces7. The walking speed depends on the lateral spacing and symmetry of the antigens. Importantly, the collision between randomly walking antibodies was seen to reduce their motional freedom. It leads to formation of transient assemblies, which are known to be nucleation sites for docking of the complement system and/or phagocytes as an important initial step in the immune cascade.
References:
- Hinterdorfer, P. et al. Proc. Natl. Acad. Sci. USA 93, 3477 (1996)
- Hinterdorfer, P. et al. Nature Methods 5, 347 (2006)
- Kienberger, F. et al. Acc. Chem. Res. 39, 29 (2006)
- Preiner, J. et al. Nanotechnology 20, 215103 (2009)
- Chtcheglova, L.A. et al. Biophys J. 93, L11 (2007)
Marco Capitanio
University of Florence, Italy
Title: High-speed optical tweezers for the study of protein-DNA interaction

Biography:
Marco Capitanio is Senior Researcher at the Department of Physics of the University of Florence, Italy, and Group Leader at the European Laboratory for Non-linear Spectroscopy (LENS). After finishing his master’s degree in physics, he gave in to his long- standing interest in biology and obtained his PhD in physiology.
He then joined LENS, a research institute which is part of a European network of laser and spectroscopy facilities.
His research interests lie across physics and biology. On one hand, his research is focused on the physics of biological systems and on the development of techniques for the study of biology at the molecular scale, with a particular interest on optical methods. On the other hand, he is particularly interested in the molecular mechanisms underlying mechanical regulation of biological systems and the conversion of mechanical signals into changes in gene expression and cell fate.
Abstract:
We developed a constant-force laser trap that allows us to investigate molecular interactions and sub-nanometer conformational changes occurring on a time scale of few tens of microseconds. [Capitanio et al., Nature Methods 9, 1013-1019 (2012)]. The method is effective in studying the sequence-dependent affinity of DNA-binding proteins along a single DNA molecule. The improvement in time resolution provides important means of investigation on the long-puzzled mechanism of target search on DNA. In fact, one poorly understood issue in the field of protein-DNA interaction is how proteins weakly interact with non-cognate DNA sequences and how they efficiently find the sequence of interest among an extremely large amount of non-specific sequences. Using our technique, we could discriminate sequence and conformational -dependent interactions of a single Lac repressor protein (LacI) on DNA at physiological salt concentrations. The lac operon is a well-known example of gene expression regulation, based on the specific interaction of LacI with its cognate DNA sequence (operator). We observed LacI switching between different interaction modalities on DNA (weak, strong, sliding), depending on the molecule conformation and DNA sequence. We provide a method for measuring 1D-diffusion constants of DNA-binding proteins along DNA with a spatial resolution of about 30 base pairs, observing a broad distribution of 1D-diffusion constants of LacI and sequence-dependent diffusion constants. Our measurements provide a model of target-search and molecular switching mechanism of Lac repressor.
References:
- Gardini, L., Capitanio, M., Pavone F.S., “3D tracking of single nanoparticles and quantum dots in living cells by out-of-focus imaging with diffraction pattern recognition”, Sci. Rep. 5, 16088; doi: 10.1038/srep16088 (2015).
- Monico, C., Belcastro, G., Vanzi, F., Pavone, F.S. and M. Capitanio, “Combining single-molecule manipulation and imaging for the study of protein-DNA interactions”, J. Vis. Exp. 90, e51446, doi:10.3791/51446 (2014).
- Capitanio, M and Pavone, F.S. “Interrogating biology with force: single-molecule high-resolution measurements with optical tweezers”, Biophys. J. 105, 1293-1303 (2013).
- Monico, C., Capitanio, M., Belcastro, G., Vanzi, F., Pavone, F.S., “Optical Methods to Study Protein-DNA Interactions in Vitro and in Living Cells at the Single-Molecule Level”, Int. J. Mol. Sci. 14, 3961-3992 (2013).
- Capitanio, M., Canepari, M., Maffei, M., Beneventi, D., Monico, C., Vanzi, F., Bottinelli, R. and Pavone F.S. “Ultrafast force-clamp spectroscopy of single molecules reveals load dependence of myosin working stroke”, Nature Methods, 9, 1013–1019 (2012).
Gijs Wuite
Vrije Universiteit Amsterdam, The Netherlands
Title: Sliding sleeves of XRCC4–XLF bridge DNA and connect fragments of broken DNA

Biography:
Gijs Wuite obtained his PhD in biophysics in 2000. Since 2001 he leads his own group at the VU University Amsterdam and in 2009 was appointed to full professor. In his research he has successfully applied quantitative physical tools to investigate fundamental problems in biology, and to search for the unification of apparently unrelated biological phenomena. Moreover, he has been at the front of recent new and fast developments of biophysical techniques that have enabled visualization, manipulation and control of complex biological reactions. Based on this research work he founded in 2014 a company (LUMICKS) that sell the technology he and his group has developed. His work has appeared in journal such as Nature, Science, PNAS and Physical Review Letters. His research has been awarded with the prestigious personal VIDI, VICI and ERC grants. In 2009 Wuite was appointed member of the Young Academy, an independent platform of young top scientists within the Royal Netherlands Academy of Arts and Sciences.
Abstract:
Non-homologous end joining (NHEJ) is the primary pathway for repairing DNA double-strand breaks (DSBs) in mammalian cells1 . Such breaks are formed, for example, during gene-segment rearrangements in the adaptive immune system or by cancer therapeutic agents. Although the core components of the NHEJ machinery are known, it has remained difficult to assess the specific roles of these components and the dynamics of bringing and holding the fragments of broken DNA together. The structurally similar XRCC4 and XLF proteins are proposed to assemble as highly dynamic filaments at (or near) DSBs2 . Here we show, using dualand quadruple-trap optical tweezers combined with fluorescence microscopy, how human XRCC4, XLF and XRCC4–XLF complexes interact with DNA in real time. We find that XLF stimulates the binding of XRCC4 to DNA, forming heteromeric complexes that diffuse swiftly along the DNA. Moreover, we find that XRCC4–XLF complexes robustly bridge two independent DNA molecules and that these bridges are able to slide along the DNA. These observations suggest that XRCC4–XLF complexes form mobile sleeve-like structures around DNA that can reconnect the broken ends very rapidly and hold them together. Understanding the dynamics and regulation of this mechanism will lead to clarification of how NHEJ proteins are involved in generating chromosomal translocations (Brouwer et al., Nature, doi:10.1038/nature18643 , 2016)
References:
- Ineke Brouwer, Gerrit Sitters, Andrea Candelli, … , Mauro Modesti, Erwin J.G. Peterman, Gijs J.L. Wuite (2016) Sliding sleeves of XRCC4-XLF bridge DNA and connect fragments of broken DNA NATURE Volume:535 Issue: 7613
- Onno D. Broekmans, Graeme A. King, Greg J. Stephens, and Gijs J.L. Wuite (2016) DNA twist stability changes with magnesium(2+) concentration PHYS. REV. LETT. Volume: 116 Issue: 25
- King, Graeme A; Peterman, Erwin J G; Wuite, Gijs J L (2016) Unravelling the structural plasticity of stretched DNA under torsional constraint NATURE COMMUNICATIONS Volume: 7 Pages: 11810
- Biebricher, Andreas S.; Heller, Iddo; … Peterman, Erwin J. G.; Wuite, Gijs J. L. (2015) The impact of DNA intercalators on DNA and DNA-processing enzymes elucidated through force-dependent binding kinetics NATURE COMMUNICATIONS Volume: 6 Article: 7304
- I Heller, G Sitters, OD Broekmans, G Farge, C Menges, W Wende, SW Hell, EJG Peterman & GJL Wuite. (2013) STED nanoscopy combined with optical tweezers reveals protein dynamics on densely covered DNA Nature Methods, 10.1038/nmeth.2599

Biography:
Dmitrii V Shalashilin is a Professor of Computational Chemistry at the University of Leeds. His research is focused on the development of efficient computational techniques for quantum and classical simulations in chemistry and their applications. The recently developed Boxed Molecular Dynamics method has a wealth of applications to structural biology and dynamics of biological molecules.
Abstract:
New applications of Boxed Dynamics (BXD) [1.2], an efficient technique to extend the time scale of molecular dynamics and simulate rare events, will be presented. BXD allows analysis of thermodynamics and kinetics in complicated molecular systems. It is a fully atomistic multiscale technique, in which thermodynamics and long-time dynamics are recovered from a set of short-time molecular dynamics simulations. BXD is many orders of magnitude faster than standard MD and can produce well converged results. Previously BXD has been applied to peptide cyclization, solution-phase organic reaction dynamics, and desorption of ions from self-assembled monolayers (SAMs) [3]. Here two new applications of BXD will be reported. First atomistic simulations of protein pulling with Atomic Force Microscope AFM) will be presented, where BXD is able to reproduce correctly the Potential of Mean Force (PMF) of a protein pulled in AFM experiments, the experimentally observed force profile and its relationship with the protein structure [4] (see Fig.1). Second, an application of BXD to enzymatic peptide cyclization will also be presented, where BXD predicts correctly the cyclizable peptide sequences [5]. All such sequences have a conformation with their C and N termini close to each other as shown at the Fig.2. In both applications calculations were done with standard force field without any adjustment of the force field parameters. Thus, BXD proves to be a good predictive tool. It is implemented in CHARMM molecular dynamics code and can be used for many other applications
Fig.1 Potential of mean force as a function of end-to-end distance calculated with BXD correlates with the structures of the unfolding protein.
Fig.2 PMF as a function of end-to-end distance for two peptides P18 and P17. Only P18, which has a stable conformation with C and N termini close to each other, is cyclizable.
References:
- Glowacki, DR; Paci, E; Shalashilin, DV (2009) Boxed Molecular Dynamics: A Simple and General Technique for Accelerating Rare Event Kinetics and Mapping Free Energy in Large Molecular Systems. Journal of Physical Chemistry 113: 16603-16611.
- Shalashilin, DV; Beddard, GS; Paci, E; Glowacki, DR (2012) Peptide kinetics from picoseconds to microseconds using boxed molecular dynamics: Power law rate coefficients in cyclisation reactions. Journal of Chemical Physics 137: 165102.
- Booth, JJ; Vazquez, S; Martinez-Nunez, E; Marks, A; Rodgers, J; Glowacki, DR; Shalashilin DV (2014) Recent applications of boxed molecular dynamics: a simple multiscale technique for atomistic simulations. Philosophical Transactions of the Royal Society A - Mathematical Physical and Engineering, 372: 20130384.
- Booth, JJ; Shalashilin, DV, (2016) Fully Atomistic Simulations of Protein Unfolding in Low Speed Atomic Force Microscope and Force Clamp Experiments with the Help of Boxed Molecular Dynamics. Journal of Physical Chemistry 120: 700-708.
- Booth, JJ; Alexandra-Crivac, CN; Rickaby, KA; Nneoyiegbe, AF; Umeobika, U; McEwan, AR;
- Track 1: Recent Advances in Structural Biology
Session Introduction
Nebojsa Janjic
SomaLogic, Inc., U.S.A.
Title: Structural insights from aptamers with base modifications
Time :

Biography:
Nebojsa Janjic has been Chief Science Officer at SomaLogic, Inc. since January 2009. Prior to joining SomaLogic, Dr. Janjic was a founder and CSO at Replidyne, Inc., a biotechnology company focusing on the development of new small-molecule antibacterial agents. Prior to Replidyne, Dr. Janjic was senior director of drug discovery at NeXstar Pharmaceuticals, where his contributions include the discovery and early development of Macugen, the first aptamer to receive FDA approval and the first VEGF inhibitor developed for the treatment for macular degeneration. As CSO at SomaLogic, Dr. Janjic is involved in developing a new generation of modified aptamers and identifying opportunities for their use in science and medicine. Dr. Janjic received his bachelor's degree in molecular biology and Ph.D. in physical organic chemistry from the University of Washington in Seattle and completed his postdoctoral training at the Scripps Research Institute in La Jolla as a Cancer Research Institute Fellow.
Abstract:
Statement of the Problem: The ability to fold into distinct three-dimensional structures is the basis of high affinity and specificity characteristic of aptamer binding to their targets. We have recently introduced base modifications that increase chemical diversity of functional groups front-loaded in randomized nucleic acid libraries from which aptamers are selected. Such modifications have allowed us to identify high-affinity aptamers to a large number of protein targets previously considered “difficult” with conventional nucleic acid libraries. At the same time, our ability to predict the structures of modified aptamers with conventional nucleic acid folding rules was severely compromised, suggesting new rules for folding. Methodology & Theoretical Orientation: We examined published structures of sixteen aptamers co-crystallized with their protein targets, including three aptamers with base modifications we reported recently. Findings: In contrast to small molecules, which are entirely encaged by aptamers, proteins present large surfaces with distinct features that are recognized by complementary surfaces on aptamers. The size of these interaction surfaces is comparable to those observed with antibodies, although for aptamers, the size range is wider on both small and large extremes. The highly flexible phosphodiester backbone allows assembly of known as well as novel nucleic acid motifs into precise three-dimensional structures that orient often discontiguous aptamer regions toward their protein targets in a manner that creates surfaces with exquisite shape complementarity. Base modifications with hydrophobic side chains allow occupancy of distinctly hydrophobic pockets on proteins and create novel structural elements that illustrate the profound role modified nucleotides play in both folding and binding. Conclusion & Significance: These observations provide compelling structural rationale for the observed high affinity and specificity with which aptamers recognize their protein targets, and show us that the lexicon of structural features accessible to nucleic acid ligands can be vastly expanded with chemical modifications of nucleic acid libraries.
References:
- Davies DR, Gelinas AD, Zhang C, Rohloff JC et al. (2012) Unique motifs and hydrophobic interactions shape the binding of modified DNA ligands to protein targets. Proc Natl Acad Sci U S A 109:19971-19976.
- Gelinas AD, Davies DR, Edwards TE, Rohloff JC et al. (2014) Crystal structure of interleukin-6 in complex with a modified nucleic acid ligand. J Biol Chem 289:8720-8734.
- Jarvis TC, Davies DR, Hisaminato A, Resnicow DI et al. (2015) Non-helical DNA Triplex Forms a Unique Aptamer Scaffold for High Affinity Recognition of Nerve Growth Factor. Structure 23:1293-1304.
- Rohloff JC, Gelinas AD, Jarvis TC, Ochsner UA et al. (2014) Nucleic Acid Ligands With Protein-like Side Chains: Modified Aptamers and Their Use as Diagnostic and Therapeutic Agents. Mol Ther Nucleic Acids 3:e201.
- Gelinas, AD, Davies, DR, Janjic, N (2016) Embracing Proteins: Structural Themes In Aptamer:Protein Complexes. Curr. Rev. Struct. Biol. 36:122-132.

Biography:
Robert Craigie is a Senior Investigator in the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health, Bethesda, MD, USA. His research has focused on the mechanism of retroviral DNA and the structure and function of proteins and nucleoprotein complexes that mediate it.
Abstract:
Statement of the problem. Integration of retroviral DNA into host DNA is an essential step in the replication of HIV-1 and other retroviruses. Integration is mediated by a nucleoprotein complex (intasome) comprising the virally encoded integrase enzyme and a pair of viral DNA ends. The first intasome on the integration pathway is the stable synaptic complex (SSC) in which a pair of viral DNA ends is bridged by integrase. Within the SSC, integrase then cleaves two nucleotides from the 3’ ends of the viral DNA to form the cleaved stable synaptic complex (cSSC). The cSSC captures a target DNA and a pair of transesterification reactions covalently joins viral to target DNA. Currently approved inhibitors of HIV-1 DNA integration target intasomes (specifically the cSSC) rather than free integrase protein, High-resolution structures of intasomes are required to understand their detailed mechanism of action and how HIV-1 can escape by acquiring resistance. Methodology and strategy: Although the structures of the individual domains of HIV-1 integrase were determined more than two decades ago, attempts to obtain high-resolution structures of HIV-1 intasomes were unsuccessful. The main obstacles were the the propensity of both integrase and intasomes to aggregate and the low efficiency of assembly in vitro. We have overcome these problems by developing a hyperactive integrase mutant that assembles intasomes that are amenable to biophysical and structural studies. CryoEM studies of STCs reveal both tetrameric and higher order species that both share a common core architecture with intasomes of related retroviruses. SSCs also assemble both tetrameric and higher order intasomes and both are active for concerted DNA integration in vitro. Conclusions and significance: The results highlight how a common core intasome architecture can be assembled in different ways. Structures of cSSC intasomes in complex with inhibitors will elucidate their detailed mechanism of action and mechanisms by which HIV-1 can evolve drug resistance.
References:
- Passos D., Li M., Yang R., Rebensburg S., Ghirlando R., Jeon Y., Shkriabai N., Kvaratskhelia M., Craigie R., Lyumkis D. (2017) CryoEM Structures and Atomic Models of the HIV-1 Strand Transfer Complex Intasome. Science, 355(6320), 89-92.
- Li, M., Jurado, K.A., Lin, S., Engelman, A., and Craigie R. (2014) Engineered hyperactive integrase for concerted HIV-1 DNA Integration. PLoS ONE Volume: 9 Issue: 8 Article Number: e105078.LiX,
- Yin, Z., Lapkouski, M., Yang, W., and Craigie, R. (2012) Assembly of prototype foamy virus strand transfer complexes on product DNA bypassing catalysis of integration. Protein Science 12, 1849-1857.
- Kotova, S., Li, M., Dimitriadis, E.K., and Craigie, R. (2010) Nucleoprotein intermediates in HIV-1 DNA integration visualized by atomic force microscopy. J. Mol. Biol. 399, 491-500.
- Li, M., Mizuuchi, M., Burke, T.R., and Craigie. (2006) Retroviral DNA integration: reaction pathway and critical intermediates. EMBO. J. 25, 1295-1304.
Dino Moras
IGBMC, Strasbourg, France
Title: Allosteric control of transcription regulation by nuclear receptors: an integrative structural biology approach

Biography:
Dino Moras, PI, graduated in chemistry at the University of Strasbourg. While post-doc with M.G. Rossmann he contributed to the concept of nucleotide binding domain known as the 'Rossmann fold'. His main scientific contributions are in structural biology, related to the expression of the genetic information: (i) translation of the genetic code by aminoacyl-tRNA synthetases: discovery of the partition of aaRS in two classes and first crystal structure of a class II tRNA-aaRS complex (ii) transcription regulation by Nuclear Receptors: the first crystal structures of the ligand binding domains of two NRs (RXR and RAR) in their apo and liganded form respectively. Presently his main focus is on the molecular mechanisms of regulation using integrative structural biology approaches.
Abstract:
Nuclear Hormone Receptors interact with corepressors, coactivators and other protein cofactors to regulate signal transduction of the basal transcriptional machinery. Most NRs are known to function as dimers and with the exception of the group of oxosteroid receptors (AR, GR, MR, PR) all structural data point to a conserved interface for the ligand binding domains (LBDs) dimers.
Allosteric mechanisms control the sequential and ordered binding of nuclear receptors to the various protein effectors and target DNA. The binding of ligands induce structural transitions in the LBDs leading to the release of the corepressors and their replacement by cofactors. The LBD swallows the ligand and shields it from the solvent by closing the pocket with the C-terminal peptide. The agonist/antagonist character of the ligand is then essentially controlled by the position of helix H12 and the stability of the complex. Ligands are modulators of the activation process, their potency being defined by the fraction of time spent in the active conformation. Crystal structures of DNA binding domains (DBDs) bound to different response elements also support the proposal of DNA being an allosteric effector. The architectures of full length receptors bound to DNA fragments and cofactors have been determined by crystallography and in solution using integrative approaches. The later combine structural small angle diffraction methods by X-Rays (SAXS) and neutrons (SANS), optical techniques like FRET with labelled molecules and single particle electron microscopy (cryo-EM). Some common features emerge that rationalize the key role of DNA. The recent advances in cryo-EM allow solution structures determination at near atomic resolution. Conformational equilibrium of NRs in complex with various cofactors are also accessible.

Biography:
John (Ioannis) Vakonakis did a PhD in Biochemistry at Texas A&M University, where he pioneered the structural analysis of bacterial circadian clock proteins. His postdoctoral work at the University of Oxford focused on the structural mechanisms underpinning cell adhesion and assembly of the extracellular matrix in animals. John did breakthrough work on the molecular architecture of the centriole organelle during a second postdoc at the Swiss Light Source, prior to starting his own lab in Oxford Biochemistry. He has been a Marie Currie Fellow, Junior Research Fellow at Trinity College, Oxford, and a Wellcome Trust Research Fellow. John is now Associate Professor in Structural Biology and Biophysics at the University of Oxford, and Fellow in Biochemistry at Lincoln College. Over the last six years his research aims to understand how large molecular machines form in cells, such as the cytoadherence assemblies created upon P. falciparum-infection of human erythrocytes.
Abstract:
Human red blood cells infected by the malaria parasite Plasmodium falciparum (iRBC) form dome-shaped ~120 nm-diameter protrusions on their surface, known as ‘knobs’. Knobs provide essential presentation platforms for the parasite cytoadherence receptor family PfEMP1, which binds ligands on endothelial cells of the blood vessel wall thereby immobilizing iRBC in the microvasculature. The resulting obstruction of blood vessels and disruption of normal circulation causes inflammation and tissue damage that can lead to coma and death. iRBC cytoadherence constitutes the primary mechanism driving morbidity and mortality in P. falciparum infections, which account for over 90% of all malaria-related deaths.
Despite their importance in malaria pathology the molecular mechanisms underpinning knob formation remain poorly understood. Here, I review recent progress in characterizing knob complexes formed between parasite and parasite – host proteins. Extensive flexibility is common among parasite knob components, which necessitated an integrative approach to resolve these complexes. In particular, I will focus on the development of novel in silico docking tools suitable for evaluating interactions between folded components and highly charged, very long and flexible protein segments. Our work offers the first glimpse of a molecular model for knobs.
References:
- Oberli A, Zurbrügg L, Rusch S, Brand F, Butler ME, Day JL, Cutts EE, Lavstsen T, Vakonakis I, Beck HP (2016) Plasmodium falciparum PHIST Proteins Contribute to Cytoadherence and Anchor PfEMP1 to the Host Cell Cytoskeleton. Cell Microbiol. 18, 1415-28.
- Warncke JD, Vakonakis I, Beck HP (2016) PHIST proteins, at the center of host cell remodeling
- Watermeyer JM, Hale VL, Hackett F, Clare DK, Cutts EE, Vakonakis I, Fleck RA, Blackman MJ, Saibil HR (2016) A spiral scaffold underlies cytoadherent knobs in Plasmodium falciparum-infected erythrocytes. Blood. 127, 343-51.
- Oberli A, Slater LM, Cutts E, Brand F, Mundwiler-Pachlatko E, Rusch S, Masik MFG, Erat MC, Beck HP, Vakonakis I (2014) A Plasmodium falciparum PHIST protein binds the virulence factor PfEMP1 and co- migrates to knobs on the host cell surface. FASEB J. 28, 4420-33.
- Boddey JA, Cowman AF (2013) Plasmodium nesting: remaking the erythrocyte from the inside out. Annu Rev Microbiol. 67, 243-69.
David F. Sargent
ETH Zurich, Switzerland
Title: Microrobotics enables non-contact, fully automated protein crystal harvesting

Biography:
David Sargent obtained his PhD in biophysics from the University of Western Ontario, Canada, followed by postdoctoral studies at the ETH Zurich and the University of Sydney (Australia). He has had extensive experience in macromolecular crystallography at the ETH Zurich, and recently has also been associated with the Multiscale Robotics Laboratory (ETH Zurich) of Bradley J. Nelson. David is one of the founders of MagnebotiX, a spinoff of the ETH, which provides tools for magnetic propulsion and guidance at the microscopic scale. The work reported here uses this technology to streamline and accelerate the process of macromolecular crystal structure determination.
Abstract:
Statement of the Problem: Most aspects of macromolecular structure determination, from synthesis and purification of materials, through crystallization, data collection and model building, are highly automated, but the recognition, harvesting and cryocooling of crystals remains a predominantly manual task. Several concepts, including in situ crystallography, are being developed to overcome these difficulties, but frequently impose other restrictions, such as on data collection strategies. We are developing hardware and software to support crystal harvesting using standard crystallization procedures, thus avoiding such limitations. Methodology & Theoretical Orientation: We use a magnetically driven, mobile, rolling microrobot, the “RodBot”, to locally move the liquid surrounding a crystal, and the crystal then passively follows the flow. Crystal position is monitored using low level uv-light. Transport is controlled using flexible algorithms that allow for error-recovery following stochastic disturbances. Findings: We demonstrate the effectiveness of the technique using crystals of different geometries and densities in a variety of buffers and cryoprotectants. Even at this developmental stage average harvesting time is reduced compared to manual operations. Conclusion & Significance: This non-destructive, non-contact method allows crystals to be extracted reliably from the growth droplet in a completely automated process. Harvesting can take place remotely in climate-controlled chambers, ensuring optimal conditions throughout the process with respect to temperature, humidity and composition of the environment. Damage to valuable crystals due to operator jitter or fatigue is eliminated. Incorporation into existing robotics setups for sample handling will also allow increased reproducibility of flash-cooling. Fully automated structure determination pipelines using well-established techniques are now possible and will yield improved data quality at reduced cost.
References:
- Zeydan B, Petruska AJ, Somm L, Pieters RS, Fang Y, Sargent DF, Nelson BJ (submitted) Automated protein crystal harvesting.
- Pieters RS, Lombriser S, Alvarez-Aguirre A, Nelson BJ (2016) Model predictive control of a magnetically guided rolling microrobot. IEEE Robotics and Automation Letters 1.1: 455-460.
- Charreyron S, Pieters RS, Tung HW, Gonzenbach M, Nelson BJ (2015) Navigation of a rolling microrobot in cluttered environments for automated crystal harvesting. Paper at IEEE/RSJ Int. Conf. Intell. Robots and Systems (IROS), Germany 2015
- Tung HW, Sargent DF, Nelson BJ (2014) Protein crystal harvesting using the RodBot: a wireless mobile microrobot. J Appl. Cryst. 47: 692-700.
- Tung HW, Peyer K, Sargent DF, Nelson BJ (2013) Noncontact manipulation using a transversely magnetized rolling robot. Appl. Phys. Letters 103.11:114101.
Toshiya Senda
High Energy Accelerator Research Organization (KEK), Japan
Title: The native (Sulfur) SAD method in Photon Factory
Biography:
Toshiya Senda has completed his PhD from Nagaoka University of Technology (Niigata, Japan) in 1995. He was a research associate in Nagaoka University of Technology (1995-2001) and a senior researcher in Institute of Advanced Industrial Science and Technology (2001-2012). Now, he is the director/professor of Structural Biology Research Center of High Energy Accelerator Research Organization (KEKI) in JAPAN. He was awarded the CrSJ (Crystallographic society of Japan) award in 2014 (Structural biology studies of CagA from Helicobacter pylori and histone chaperon CIA/ASF1).
Abstract:
Crystallography has been a major method to determine 3D structures of biological macromolecules at atomic resolution. While a new method with cryo-EM is becoming another major technique for the 3D structure analysis, crystallography still has some advantages. Recently, many crystal structures of biological macromolecules are determined by MAD/SAD method with seleno-methionine proteins (SeMet-proteins). Since selenium has an X-ray absorption edge near 1Å, it is convenient to utilize in the MAD/SAD method. While this technique is useful, crystallographers need to prepare SeMet-proteins. If we can develop a method to solve the phase problem without using SeMet-proteins, it would be highly useful for crystallographers. So, we have tried to develop the native SAD (or sulfur SAD) method, in which anomalous signals from sulfur atoms in the native protein are utilized. However, there are some problems in the native SAD method. First, sulfur gives only weak anomalous signals with X-ray typically utilized in protein crystallography (X-ray wavelength of around 1Å). To increase the anomalous signals, we need to use a longer wavelength X-ray than usual. However, since a longer wavelength X-ray shows significant absorption by air, solvent, protein etc., data quality is degraded by the absorption. The native SAD method, therefore, requires a specific system for high quality data collection. To achieve routine utilization of the native SAD method, we have developed a beamline (BL-1A) dedicated for the native SAD method. In BL-1A, we can utilize a long wavelength X-ray (1.9 – 3.5 Å). Furthermore, the goniometer and X-ray detector are installed inside a He chamber to prevent the absorption problem. Our system enables us to solve crystal structures of proteins by the native SAD method. In the presentation, we will present several examples of crystal structure determination with native SAD. Also, we will mention our unique method for crystal freezing to collect high quality diffraction data required in native SAD experiments.
References:
- Liebschner, D., Yamada, Y., Matsugaki, N., Senda, M. & Senda, T. (2016). On the influence of crystal size and wavelength on native SAD phasing. Acta Crystallogr. D72, 728–741.
- Hiraki, M., Matsugaki, N, Yamada, Y. & Senda, T. (2016) Development of sample exchange robot PAM-HC for beamline BL-1A at the photon factory. API Conf. Proc. 1741, 030029.
- Nagae, M., Liebschner, D., Yamada, Y., Morita-Matsumoto, K., Matsugaki, N., Senda, T., Fujita, M., Kinoshita, T., Yamaguchi, Y. (2017) Crystallographic analysis of murine p24g2 Golgi dynamics domain. Proteins, 85, 764-770. doi: 10.1002/prot.25242
- Nagae, M., Mishra, S. K., Neyazaki, M., Oi, R., Ikeda, A., Matsugaki, N., Akashi, S., Manya, H., Mizuno, M., Yagi, H., Kato, K., Senda T., Endo, T., Nogi, T. & Yamaguchi Y. (2017) Genes to Cells, doi 10.1111/gtc.12480 [Epub ahead of print]
- Senda, M., Hayashi, T., Hatakeyama, M., Takeuchi, K., Sasaki, A. T. & Senda, T. (2016) Use of multiple cryoprotectants to improve diffraction quality from protein crystals. Crystal Growth & Design 16, 1565-1571. Doi: 10.1021/acs.cgd.5b01692
Yawen Bai
NIH, USA
Title: Structural mechanisms of nucleosome recognition as revealed by methyl-TROSY
Time :

Biography:
Yawen Bai received his Ph.D. in Biophysics from the University of Pennsylvania Medical School. After postdoctoral work at the Scripps Research Institute, La Jolla, California, he became an investigator at the National Cancer Institute of the National Institutes of Health in Bethesda, Maryland since 1997. The research interests of his group include structural studies on protein folding intermediates, histone chaperones, epigenetic specification of centromeres and chromatin folding.
Abstract:
Human genome is packaged into chromatin through association with small positively charged histone proteins. The structural unit of chromatin is the nucleosome, which consists of ~147 bp of DNA and two copies of each of the four core histones (H2A, H2B, H3 and H4). Numerous proteins regulate chromatin structure and function through specific binding to the nucleosome. The structural basis of many of these interactions is unknown. Structural determination of the nucleosome in complex with a protein by X-ray crystallography and single particle cryo-EM has proven to be very challenging in many cases due to difficulties to crystalize them and dissociation of the complex during cryo processes. On the other hand, the nucleosome is too large (> 200 KDa) for structural studies with conventional NMR methods. We have used methyl-TROSY [1] coupled with site-specific mutagenesis and paramagnetic spin labeling to investigate how the nucleosome is recognized by various chromatin factor proteins, including high-mobility group nucleosomal protein [2], centromere protein C [3] and linker histones [4,5]. Major results and future perspectives will be presented.
References:
- Tugarinov V, Hwang JE, Ollerenshaw JE, Kay LE. (2003) Cross-correlated relaxation enhanced 1H-13C NMR spectroscopy of methyl groups in very high molecular weight proteins and protein complexes. J Am Chem Soc 125, 10420-10428.
- Kato H, van Ingen H, Zhou BR, Feng H, Bustin M, Kay LE, Bai Y. (2011) Architecture of the high mobility groups nucleosomal protein 2-nucleosome complex as revealed by methyl-based NMR. Proc Natl Acad Sci 108, 12283-12288.
- Kato H, Jiang J, Zhou BR, Rozendaal M, Feng H, Ghirlando R, Xiao TS, Straight AF, Bai Y. (2013) A conserved mechanism for centromeric nucleosome recognition by centromere protein CENP-C. Science, 340, 1110-1113.
- Zhou BR, Feng H, Kato H, Dai L, Yang Y, Zhou Y, Bai Y. (2013) Structural insights into the histone H1-nucleosome complex. Proc Natl Acad Sci 110, 19390-19395.
- Zhou BR, Jiang J, Feng H, Ghirlando R, Xiao TS, Bai Y. (2015) Structural mechanisms of nucleosome recognition by linker histones. Mol Cell 58, 628-638.
Biography:
Julien Boudet received his PhD degree in structural biology and biophysics from the University of Grenoble (Joseph Fourier University) in France under the supervision of Prof. Jean-Pierre Simorre. During his thesis, he learned nuclear magnetic resonance (NMR) spectroscopy and used this powerful method to investigate proteins and oligonucleotides structures, molecular mechanisms underlying antibiotic resistance and viral proteins interactions. After graduating, Julien joined the group of Prof. Frédéric Allain in ETH Zurich as a researcher. He focused his investigations on the DNA replication machinery and, in particular on the primase-mediated catalysis. He set up innovative computational methods to investigate challenging biological systems and demonstrated the role of cofactors in improving the pRN1 primase specific template recognition. His currently developing computational and analysis tools for structural biology.
Abstract:
Primases are single-stranded DNA dependent polymerases that synthesize RNA/DNA primers during replication. A primase, a DNA polymerase and an helicase compose the replication machinery of the archaeal plasmid pRN11. The structure of the archaeal functional primase domain has been solved by X-ray crystallography 2,3 and it revealed an heteromeric structure with a catalytic prim/pol domain tethered to a novel helix bundle domain.
We investigated the NMR structure of the functional pRN1 primase domain in complex with a single-stranded DNA template containing the GTG motif 4. We showed that the catalytic prim/pol domain of this 38 kDa enzyme is not required for template binding. Intermolecular contacts detected exclusively between the helix bundle domain and the DNA led us to isolate specifically this structurally independent unit. Our results are compatible with a conformational switch between a template-bound open state and a closed active complex 3,5,6.
We solved the solution structures of the helix bundle domain in complex with the single-stranded DNA template alone and upon cofactors addition. Affinity measurements validated our structural data demonstrating the importance of residues located in helices 10 and 12 for the interaction with the GTG motif and confirmed the specificity improvement observed upon cofactors binding.
In association with functional assays, these novel transient structures bring new perspectives and will help us to characterize the molecular steps required for priming.
References:
- Lipps G., Röther S., Hart C. and Krauss G. (2003) EMBO J, 22, 2516-25.
- Lipps G., Weinzierl AO., von Scheven G., Buchen C. and Cramer P. (2004) Nat. Struct. Mol. Biol., 11, 157-62.
- Beck K., Vannini A., Cramer P. and Lipps G. (2010) Nucleic Acids Res., 38, 6707-18.
- Beck K. and Lipps G. (2007) Nucleic Acids Res., 35, 5635-45.
- Lipps G. (2011) Biochem Soc Trans., 39, 104-6
Luis Paulo. B. Scott
Universidade Federal do ABC , Brazil
Title: A new protocol to investigate conformational population patterns in the enzymatic activity cycle of proteins using molecular dynamics and normal mode analysis

Biography:
Luis Paulo Barbour Scott is Associate Professor in Federal University of UAFBC. He has his expertise in conformational changes and functional movements of macromolecules, specially proteins. Over the last four years, Dr. Luis Paulo Scott’s research group has been financed to investigate molecules related to neurodegeneration and aggregate formation by means of normal mode analysis and molecular dynamics combined. The laboratory coordinated by Dr. Luis Paulo Scott has become more and more specialized in the study of macromolecules structural dynamics (functional movements in collaboration with Dr. David Perahia from France.
Abstract:
Human immunodeficiency virus type-1 protease (HIV-1 PR) is an aspartic protease whose proteolytic activity is essential for cleaving precursor viral polyproteins into individual proteins implied in viral replication. Once HIV enters within a host cell, its RNA is transcribed into DNA through reverse transcriptase, integrated and amplified along with the replication of the host cell's DNA. Gag and gag-pro-pol genes are transcribed into messenger RNA, translated into gag and gag-pro-pol precursors proteins in the cytoplasm, and then assembled at the cell surface for budding and formation of the immature viral particles. In this work, we propose a computational protocol to generate and select HIV protease conformations relevant to its function using Normal Mode Analysis (NMA). We have considered structures of the apoenzyme, the protein with its substrate and product and the protein with a drug. This set of structures should reveal large amplitude motions that are critical to the protease activity cycle as: i) substrate acquisition; ii) substrate cleavage and; iii) product release. The apoenzyme presents an increased flap conformational diversity compared to the various complexes, predominantly populated with open flap conformations, that can possibly be related to the substrates acquisition. The enzyme-substrate complexes show more structural diversity than enzyme-product complexes, suggesting a role of these conformational changes in catalytic activity. We presents a promising protocol to identify the conformational diversity induced by different types of ligands and that can help the drug design process.
References:
- Lima, A N. ; Philot, E. A. ; TROSSINI, G. ; SCOTT, L. P. B. ; MALTAROLLO, V. ; HONORIO, K. M. . Use of machine learning approaches for novel drug discovery. Expert Opinion on Drug Discovery (Print), v. 11, p. 225-239, 2016.
- MEISSNER, G. ; RESENDE-LARA, P. ; MATSUBARA, F. ; Luis P B Scott ; BRAZ, ANTÔNIO S.K. ; CHAVES-MOREIRA, D. ; SOARES, E. ; TREVISAN-SILVA, D. ; GREMSKI, L. ; VEIGA ; CHAIM, O. . Molecular cloning and in silico characterization of knottin peptide, U2-SCRTX-Lit2, from brown spider (Loxosceles intermedia) venom glands. Journal of Molecular Modeling (Online), v. 22, p. 196, 2016.
- ARAUJO, G. ; Silva, R H T ; SCOTT, L. P. B. ; ARAUJO, A. S. ; SOUZA, F. P. ; OLIVEIRA, R. J. . Structure and functional dynamics characterization of the ion channel of the human respiratory syncytial virus (hRSV) small hydrophobic protein (SH) transmembrane domain by combining molecular dynamics with excited normal modes. Journal of Molecular Modeling (Print) , v. 22, p. 3-8, 2016.
- Philot, E. A. ; LOPES, D. M. ; SOUZA, A. T. ; BRAZ, A. S. K. ; NANTES, I. L. ; RODRIGUES, T. ; Perahia, D. ; MITEVA, M. A. ; SCOTT, L. P. B. . Binding of phenothiazines into allosteric hydrophobic pocket of human thioredoxin 1. European Biophysics Journal, v. 43, p. 2798-286, 2016.
- CONTO, V. ; BRAZ, A. S. K. ; Perahia, D. ; SCOTT, L. P. B. . Recovery of the wild type atomic flexibility in the HIV-1 protease double mutants. Journal of Molecular Graphics & Modelling , v. 59c, p. 107-116, 2015.
Thomas Braun
University of Basel, Switzerland
Title: Cryo-Electron Microscopy Grid Preparation from Nanoliter-Sized Protein Samples and Single-Cell Extracts

Biography:
Thomas Braun received his Ph.D. 2002 in biophysics from the Biozentrum, University of Basel, Switzerland. During his Ph.D. thesis he applied high-resolution electron microscopy and digital image processing to study the structure and function of membrane proteins. Subsequently, he worked on nanomechanical sensors to characterize the mechanics of membrane proteins at the Institute of Physics, University Basel and the CRANN, Trinity College Dublin, Ireland. He has been working at the Center for Cellular Imaging an NanoAnalytics (Biozentrum, University of Basel, Switzerland) since 2009 and is developing new methods for electron microscopy, single cell analysis and nanomechanical sensors for biological applications.
Abstract:
Cryo-electron microscopy (cryo-EM) sample preparation techniques ensure that biological specimens can be investigated at physiological conditions in the electron microscope. However, these preparation methods suffer from extensive blotting steps leading to a massive loss of sample and sometimes to partial denaturation of sensitive protein complexes. We have developed a simple method for the almost lossless conditioning and preparation of nanoliter-volumes of biological samples for EM. The method does not involve any blotting steps. A microcapillary is used to aspirate 3 to 20 nanoliters of sample, depending on the experiment (Figure 1A). The sample is applied (left) and spread (center) on the EM-grid. Real-time monitoring allows the thickness of the water film to be assessed and decreased to the optimum value prior to vitrification (right). We prepared cryo-EM grids of various samples, e.g., bacteriophages and soluble proteins as shown in Figure 1B&C, to demonstrate the usefulness and general applicability of the method. We also showed that high-resolution 3D structures can be calculated from single-particle preparations of a soluble protein. In addition to cryo-EM grid preparation, the versatile method allows nanoliter-sized sample volumes to be conditioned for EM, e.g., negatively stained with heavy metal salts or embedded in trehalose.
In addition, we combine the new sample preparation method with a single cell lysis device for adherent eukaryotic cells and image the aspirated cell contents by TEM. To demonstrate the usefulness of this new “visual proteomics” approach we visualized the changes occurring in single cell proteomes upon heat shocking the cells. Furthermore, we have developed a protein-fishing method based on a magnetic trap and photo-cleavable composite material, to ‘fish’ untagged proteins from cell lysate by antibodies. This allows target proteins to be isolated from approx. 40’000 cells in 90 min and analysed by EM.
References:
- Kemmerling S, Ziegler J, Schweighauser G, Arnold S A, Giss D, Müller S A, Ringler P, Goldie K N, Goedecke N, Hierlemann A, Stahlberg A, Engel A, Braun T (2012) Connecting µ-fluidics to electron microscopy. Journal of Structural Biology, 177:1, 128–134.
- Arnold S A, Albiez S, Opara N, Chami M, Schmidli C, Bieri A, Padeste C, Stahlberg H, Braun T (2016) Total sample conditioning and preparation of nanoliter volumes for electron microscopy. ACS Nano, 10: 5, 4981–4988.
- Arnold S A, Albiez S, Bieri A, Syntychaki, A, Adaixo R, McLeod R A, Goldie K N, Stahlberg H, Braun T. (2016) Blotting-free and lossless cryo-electron microscopy grid preparation from nanoliter-sized protein samples and single-cell extracts. Journal of Structural Biology, in press.
- Kemmerling S, Arnold S A , Bircher B, Sauter N, Escobedo C, Dernick G, Hierlemann A, Stahlberg H, Braun T (2013) Single-cell lysis for visual analysis by electron microscopy. Journal of Structural Biology, 183: 3, 467–473.
- Giss D, Kemmerling S, Dandey V, Stahlberg H, Braun T (2014) Exploring the interactome: microfluidic isolation of proteins and interacting partners for quantitative analysis by electron microscopy. Analytical Chemistry, 86: 10, 4680–4687.
- Track 2: Molecular Modeling and Drug Designing
Location:
Session Introduction
Michael Hennig
leadXpro AG, Villigen, Switzerland
Title: Structure Based Drug Discovery on Membrane Protein Targets: New developments and advancements

Biography:
Michael Hennig is a drug discovery research manager with 22 years of experience in pharmaceutical industry. He co-founded and is CEO and Chairman of the board of leadXpro, an emerging biotech company and spin-out of the Paul Scherrer Institute (ETH, Switzerland) that is dedicated to structure based drug discovery of membrane protein targets. Formerly he worked 20 years at Roche research Basel, Global Head and Principle Leader of discovery technologies with responsibility for structure based drug discovery, protein science, assay development and HTS, corporate compound library, stem cell platform. In addition, he is guest Professor at the University of Basel in structural biology, gives lecture series in pharmacy, is author of >75 paper and lecturer at conferences, inventor of 8 patents in areas of technology, discovery and formulation of drug substances.
Abstract:
Today, structure based drug discovery is well implemented in the drug discovery engine of many pharmaceutical companies. Whereas soluble proteins are managed well within the project timelines and portfolio changes in pharmaceutical industry, transmembrane proteins still represent a significant challenge. LeadXpro combines expertise of drug discovery, excellence in high quality solubilized and purified membrane protein science and use of cutting edge biophysical methods like X-ray data collection at synchrotron and FEL sources, single particle cryo-electron microscopy, SPR and others. Strong relationship between leadXpro and Swiss large research facilities like PSI-SLS and SwissFEL as well as C-CINA will enable advances in structure determination of challenging membrane protein drug targets that have not been feasible before. Knowledge of the drug candidate and protein target 3D-structure, together with the full characterization of its interaction by biophysical binding and functional assays will enable to generate novel and better lead molecules for future medicines.
Examples of recent developments include the successful fragment screening for the GPCR neutrotensin receptor 1, a fragment screening with 6369 compounds was performed with SPR and 44 hits identified. Finally, 4 selected hits were validated in NMR experiments and computational analysis gave insight into the potential fragment-binding location and interactions to inspire further chemistry efforts. Furthermore, serial crystallography performed at synchrotron and free electron laser enables structure determination on challenging drug targets. Advantages are analysis at physiologically more relevant room temperature (no freezing of crystals required), low or no radiation damage and the use of very small crystals.
References:
- Renaud, J-P., Chung, C-W., Danielson, U.H., Egner, U., Hennig, M., Hubbard, R.E., Nar, H. Biophysics in drug discovery : impact, challenges and opportunities. Nature Reviews Drug Discovery 15, 679-698 (2016)..
- Huber, S., Casagrande, F., Hug, M.N., Wang, L., Heine, P., Kummer, L., Plückthun, A., Hennig, M. SPR-based fragment screening with neurotensin 1 generates novel small molecule ligands. PlosONE, 2017
- Bocquet, N., Kohler, J; Hug, M.N., Kusznir, E., Rufer, A.C.,Dawson, R.J., Hennig, M., Ruf, A, Huber, W., Huber, S., Real time monitoring of binding events on a thermostabilized human A2A receptor embedded in a lipid bilayer by surface plasmon resonance. BBA- Biomembranes, 2015, Biochim. Biophys Acta 1848 (5), 1224-1233 (2015).

Biography:
Dirksen Bussiere Led 22-FTE structural biology and biophysics research effort (X-ray crystallography, biophysics, NMR and protein biochemistry support) on multiple structure-based projects encompassing variety of anti-infective, metabolic, and oncology targets
Led fragment-based screening and biophysics team which spans four functional areas (Structural and Biophysical Chemistry, Computational Chemistry, Biochemical Lead Discovery, and Protein Sciences)
Led multiple hit-finding project teams focused on finding hits for anti-infective and oncology
targets
Served as lead structural biologist. biochemist and biophysicist on multiple oncology and anti-infective drug discovery project teams
Provided molecular modeling, drug design, and bioinformatics support to project teams
Provided fragment-based screening support for project teams, including NMR and biophysical screening
Named Novartis Leading Scientist in 2007, an award & title given to less than 1% of Novartis scientists world-wide
Abstract:
Several biological functions, particularly chromosome segregation, require the generation of motile force. The generation of this force relies heavily on a class of proteins known as motor proteins. Motor proteins such as Kinesin Spindle Protein (KSP), also known as Eg5, utilize the energy derived from ATP hydrolysis to generate motile force. High-throughput screening of Eg5 identified several hits which were non-competitive with ATP with micromolar IC50’s capable of inhibiting the motor protein. Using structure-based drug design, these hits were progressed to NVP-BQS481, a clinical candidate with an IC50 of 700 picomolar. The talk will present the design concepts and optimization techniques used to advance the series to the pre-clinical stage.
References:
- Barsanti, PA, Wang, W, Duhl, D, Brameier, N, Martin, E, Bussiere, D, Walter AO (2010) The discovery of tetrahydro-beta-carbolines as inhibitors of the kinesin Eg5. Bioorg Med Chem Lett 20(1): 157-160.
- Murray, JM, Bussiere DE (2009) Targeting the purinome. Methods Mol Biol 575: 47-92.
- Mayer, TU, Kapoor, TM, Haggarty, SJ, King RW, Schreiber, SL, Mitchison, TJ (1999) Small molecule inhibitor of mitotic spindle bipolarity identified in phenotype-based screen. Science 286: 971-974.
- Duhl, DM, Renhowe PA (2005) Inhibitors of kinesin motor proteins—research and clinical progress. Curr Opin Drug Discov Devel 8(4): 431-436.
- Bergnes, G, Brejc, K, Belmont, L (2005) Mitotic kinesins: prospects for antimitotic drug discovery. Curr Top Med Chem 5(2): 127-145.
Christina Scharnagl
Technical University of Munich, Germany
Title: Does the dynamics of their transmembrane domain qualify bitopic membrane proteins as substrates for intramembrane proteolysis?

Biography:
Christina Scharnagl has her expertise in molecular dynamic simulations of membrane proteins. Her work focuses on biophysical principles of the interdependence of transmembrane helix dynamics, helix-helix binding, and helix-lipid interactions. In silico modelling and advanced computational analysis are closely connected to experimental work in research collaborations in order to interpret and guide the experiments and to validate the simulations. The aim of the joint efforts is to understand the impact of these phenomena on multiple biological processes, such as membrane fusion and intramembrane proteolysis.
Abstract:
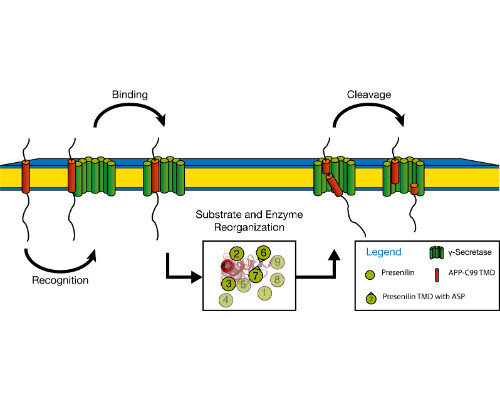
Integral membrane proteins facilitate communication between the inside of the cell and its exterior. Their transmembrane domains (TMDs) support a diversity of biological functions and exhibit sequence-dependent conformational dynamics on multiple size and time scales. Membrane proteins are notoriously difficult to study by experimental methods. Molecular dynamics (MD) simulations provide a powerful tool of high spatial and temporal resolution that effectively complements experimental methods. Here we focus on the conformational dynamics of the TMD of the amyloid precursor protein (APP). APP is enzymatically hydrolyzed within its TMD by g-secretase (GSEC), forming toxic Ab peptides regarded as molecular cause of Alzheimer's disease (AD). Besides APP, GSEC cleaves ~100 single-span membrane proteins within their TMDs, however without obvious consensus sequence. Finding the link between the molecular architecture of the substrate TMDs and cleavage is, therefore, of upmost importance. Because unfolding is obvious to expose the scissile bond, it seems plausible that the TMD itself is optimized for local helix unwinding. However, this view was challenged by our experiments and MD simulations. Our results suggest an alternative model where reaching a cleavage-competent state involves multiple conformational transitions of the substrate/enzyme complex where global conformational plasticity of the substrate TMD is a key determinant. In a first step, we compare the conformational flexibility of a large number of substrate and non-substrate TMDs, as well as TMDs carrying missense mutations related to early onset AD. Knowing the key-dynamical motifs will help to identify new substrates and to elucidate the physiological functions of the protease in the brain and other organs. This work is part of a collaborative research program (https://www.i-proteolysis.de/).
References:
- Langosch C, Scharnagl C, Steiner H, Lemberg M (2015) Understanding intramembrane proteolysis: from protein dynamics to reaction kinetics. Trends Biochem. Sci. 40:318-327.
- Scharnagl C, Pester O, Hornburg P, Hornburg D, Götz A, Langosch D (2014) Side-chain to main-chain hydrogen bonding controls intrinsic backbone dynamics of the amyloid precursor protein transmembrane helix. Biophys. J. 106: 1318-1329.
- Pester O, Götz A, Multhaup G, Scharnagl C, Langosch D (2013) The cleavage domain of the amyloid precursor protein transmembrane helix does not exhibit above-average backbone dynamics. ChemBioChem 14:1943-1948.
- Pester O, Barrett P, Hornburg D, Hornburg P, Pröbstle R, Widmaier S, Kutzner C, Dürrbaum M, Kapurniotu A, Sanders CR, Scharnagl C, Langosch D (2013) The backbone dynamics of the amyloid precursor protein transmembrane helix provides a rationale for the sequential cleavage mechanism of g-secretase. J. Am. Chem. Soc. 135: 1317-1329.
- Ried C, Scharnagl C, Langosch D (2015) Entrapment of water at the transmembrane helix-helix interface of quiescin sulfhydryl oxidase 2. Biochemistry 55: 1287-1290.
Andreas Kuhn
University of Hohenheim, Germany
Title: The dynamics of a protein during its insertion into a membrane

Biography:
Andreas Kuhn has his expertise in protein folding of membrane proteins. Studies include reconstituted systems with bacterial translocases and insertase, as well in vivo studies with Escherichia coli. For biophysical experiments the membrane proteins are purified and their folding is monitored spectroscopically in real time after their addition to liposomes. Andreas Kuhn obtained his PhD from the Universities Basel and Freiburg i. Br. 1982. After a postdoc at UCLA with Bill Wickner he continued at the Biozentrum Basel from 1986 to 1989 and accepted a professorship at the University Karlsruhe. Since 1996 he is at the University of Hohenheim in Stuttgart.
Abstract:
Most membrane proteins are inserted co-translationally by the Sec-translocase or the YidC/Oxa1/Alb3 insertases. The folding of these proteins occurs within the membrane during the interaction with the insertases. We have purified and reconstituted YidC, the membrane insertase of Escherichia coli. The protein spans the membrane 6 times, and the recently solved structure shows a hydrophilic cavity and a greasy slide between the transmembrane segments TM3 and TM5. Hydrophobic residues of TM3 and TM5 interact with the substrate, in particular with a prospective transmembrane segment of an inserting membrane protein as we documented by disulfide crossinking experiments.
The membrane insertion process can be studied with the reconstituted vesicle system. The purified substrate proteins are solubilized in 10% isopranol or kept unfolded with urea or GuHCl. When the substrate proteins are added to the proteoliposomes by dilution 1:100, they rapidly bind to YidC and become membrane inserted within 2 msec. FRET-based kinetic measurements show that the substrate proteins approach YidC to a close distance during the insertion event. Time-resolved fluorenscence anisotropy shows that the periplasmic domain of YidC moves when a substrate protein was added. This suggests that both the insertase and the substrate protein undergo conformational motions.
References:
- Winterfeld, S., S. Ernst, M. Börsch, U. Gerken and A. Kuhn (2013). Real time observation of single membrane protein insertion events by the E. coli insertase YidC. PLOSone 8, e59023.
- Dalbey, R.E. and A. Kuhn A. (2014) How YidC inserts and folds proteins across a membrane. Nature Struc.& Mol.Biol. 21, 435-436.
- Dalbey, R. E. and A. Kuhn (2015). Membrane insertases are present in all three domains of life. Structure 23, 1559-1560.
- Proß, E., L. Soussoula, I. Seitl, D. Lupo and A. Kuhn (2016). Membrane targeting and insertion of the C-tail protein SciP. J. Mol. Biol. 428, 4218-4227.
- Kuhn, A. and R.E. Dalbey (2016). A conformational crosstalk between SecA and SecY opens the SecYEG channel. Current Biol. 26, 811-813.
Shuanghong Huo
Clark University, USA
Title: Extract the thermodynamic and kinetic information from protein simulations using dimensionality reduction

Biography:
Shuanghong Huo received her Ph.D. in Computational Chemistry from Boston University. She had postdoctoral training at UC-San Francisco. She is a Professor of Chemistry and Biochemistry at Clark University, Worcester, USA. Her research interest is protein folding, misfolding, and aggregation. Recently her group is developing dimensionality reduction methods and graph representations of protein free energy landscapes.
Abstract:
In the study of protein thermodynamics and kinetics it is of paramount importance to characterize protein free energy landscapes. Dimensionality reduction is a valuable tool to complete the task. We have evaluated several methods of dimensionality reduction, including linear and non-linear methods, such as principal component analysis, Isomap, locally linear embedding, and diffusion maps. A series of criteria was used to assess different aspects of the embedding qualities. Our results have shown that there is no clear winner in all aspects of the evaluation and each dimensionality-reduction method has its limitations in a certain aspect. The linear method, principal component analysis, is not worse than the nonlinear ones in some aspects for our peptide system. We have also developed a mathematical formulation to demonstrate that an explicit Euclidean-based representation of protein conformation space and the local distance metric associated to it improve the quality of dimensionality reduction. For a certain sense, clustering protein conformations into macro-clusters to build a Markov state model is also an approach of dimensionality. We have tested inherent structure and geometric metric for state space discretization and demonstrated that the macro-clusters based on inherent structure give a meaningful state space discretization in terms of conformational features and kinetics.
References:
- H. Liu, M. Li, J. Fan and S. Huo (2016) Inherent Structure Versus Geometric Metric for State Space Discretization. J. Compt. Chem. 37:1252-1258.
- M. Duan, H. Liu, M. Li, L. Han and S. Huo (2015) Network representation of conformational transitions between hidden intermediates of Rd-apocytochrome b562. J. Chem. Phys. 143: 135101-10.
- M. Duan, M. Li, L. Han, and S. Huo (2014) Euclidean sections of protein conformation space and their implications in dimensionality reduction. Proteins: Structure, Function, and Bioinformatics. 82: 2585-2596.
- M. Li, M. Duan, J. Fan, L. Han and S. Huo (2013) Graph representation of protein free energy landscape. J. Chem. Phys. 139: 185101-8.
- M. Duan, J. Fan, M. Li, L. Han and S. Huo (2013) Evaluation of Dimensionality-Reduction Methods from Peptide Folding–Unfolding Simulations. J. Chem. Theory Comput. 9: 2490-2497.
Marek Cieplak
Institute of Physics PAS, Poland
Title: Dynamics of knotted and entangled neurotoxic polypeptides

Biography:
Head, Laboratory of Biological Physics, Institute of Physics, Polish Academy of Sciences in Warsaw, Poland. Education - M.S., Department of Physics, University of Warsaw, 1973; Ph. D., Department of Physics, University of Pittsburgh, 1977; D.Sc., Department of Physics, University of Warsaw, 1984. Professorial title, 1994. Fields of interest – condensed matter theory (spin waves, spin glasses, porous media, growth processes, atomic friction, river networks, nanofluidics, self-organized nanostructures) and biological physics (large conformational changes of biomolecules within coarse-grained models, especially as induced by stretching, proteins with knots and slipknots, protein folding, dynamics of virus capsids and other multi-proteinic structures such as a cellulosome, interaction of proteins with solids, proteins at air-water interface, modeling of proteasomes, inference of genetic networks from the microarray data). Co-author of textbook “Theory of Quanta”, Oxford University Press 1992. 250 research papers.
Abstract:
We review the physics of processes involving large conformational transformations in knotted proteins in bulk water and then consider folding in ribosomes and unfolding in proteasomes. Formation of a knot is demonstrated to be facilitated by the nascent conditions at the ribosome. Knots in proteins have been proposed to resist proteasomal degradation. Ample evidence associates proteasomal degradation with neurodegeneration. One interesting possibility is that indeed knotted conformers stall this machinery leading to toxicity. However, although the proteasome is known to unfold mechanically its substrates, at present there are no experimental methods to emulate this particular traction geometry. Here, we consider several dynamical models of the proteasome in which the complex is represented by an effective potential with an added pulling force. This force is meant to induce translocation of a protein or a polypeptide into the catalytic chamber. The force is either constant or applied periodically. The translocated proteins are modelled in a coarse-grained fashion. We do comparative analysis of several knotted globular proteins and the transiently knotted polyglutamine tracts of length 60 alone and fused in exon 1 of the huntingtin protein. Huntingtin is associated with Huntington disease, a well-known genetically-determined neurodegenerative disease. We show that the presence of a knot hinders and sometimes even jams translocation. We demonstrate that the probability to do so depends on the protein, the model of the proteasome, the magnitude of the pulling force, and the choice of the pulled terminus. In any case, the net effect would be a hindrance in the proteasomal degradation process in the cell. This would then yield toxicity via two different mechanisms: one through toxic monomers compromising degradation and another by the formation of toxic oligomers.
References:
- Zhao Y, Chwastyk M, Cieplak M (2017) Topological transformations in proteins: effects of heating and proximity of an interface. Sci. Rep. 7:39851alcohol-related braindamage. Alcohol Alcohol 44:136-140.
- Wojciechowski M, Cieplak M (2016) Dual binding mode in cohesin-dockerin complexes as assessed through stretching. J. Chem. Phys. 145:134102.
- Wolek K, Cieplak M (2016) Criteria for folding in structure-based models of proteins. J. Chem. Phys. 144:185102.
- Wojciechowski M, Gomez-Sicilia A, Carrion-Vazquez M, Cieplak M (2016) Unfolding knots by proteasome-like systems: simulations of the behavior of folded and neurotoxic proteins. Mol. Biosyst. 12:2700-2712.
- Gomez-Sicilia A, Sikora M, Cieplak M, Carrion-Vazquez M (2015) An exploration of the universe of polyglutamine structures. PLOS Comp. Biol. 11:e1004541.

Biography:
Chantal Prévost is researcher at the Theoretical Biochemistry Laboratory (LBT) of the French National Research Center (CNRS), in Paris. She has developed a large expertise in studying macromolecular self-assembliy in silico, either by elaborating new algorithms for flexible proteins docking or by studying fundamental biological processes involving the transition between instable conformational substates. She presently applies this expertise to exploring the architecture or oligomeric assemblies as well as elucidating the mechanism of homologous recombination, in collaboration with experimental partners.
Abstract:
Statement of the Problem: Many proteins present highly flexible or disordered fragments, either terminal tails or surface loops. Although they often form instable and transient interactions, these fragments play essential roles in regulating macromolecular association or controlling the architecture of supramolecular complexes. The role of their conformational variability in complex formation is poorly understood and requires the development of specific approaches.
Methodology & Theoretical Orientation: We have studied the effect of protein segment conformational variability in protein-protein complex formation as well as peptide docking using theoretical docking approaches. Notably, we have developed a flexible docking method that accounts for the presence of flexible loops, together with analysis protocols that capture the entropic effects associated to structural variability in flexible docking results.
Findings: Whether the flexible segment is a loop or a peptide, we have found that a given mode of association can be stabilized by different conformations of the segment. Alternatively, different loop conformations can stabilize different modes of protein-protein association.
Conclusion & Significance: Tolerance of a binding site to conformational variability, as observed in protein-peptide docking but also in the association of proteins with flexible loops or segments, can play a role in adding a conformational entropy component to the energy of association, thus favoring the initial binding of the flexible fragment to its binding site. For proteins that associate using different binding geometries, either with different partners or along a functional pathway, loop flexibility can also be used to regulate the choice of the binding geometry.
References:
- Sacquin-Mora S, Prévost C (2015) Docking peptides on proteins: How to open a lock, in the dark, with a flexible key. Structure, 23:1373-1374.
- Laurin Y, Savarin P, Robert C, Takahashi M, Eyer J, Prévost C, Sacquin-Mora S (2015) Investigating the binding modes of the glioma targeting NFL-TBS.40-63 peptide on tubulin via docking and molecular dynamics simulations. Biochemistry 54:3660-3669.
- Bastard K, Thureau A, Lavery R and Prévost C (2003) Docking macromolecules with flexible segments. Journal of Computational Chemistry, 24:1910-1920.
- Bastard K, Prévost C and Zacharias M (2006) Accounting for loop flexibility during protein-protein docking. Proteins: Structure, Function and BioInformatics, 62:956-969
- Boyer B, Ezelin J, Poulain P, Saladin A, Zacharias M, Robert CH, Prévost C (2015). An integrative approach to the study of filamentous oligomeric assemblies, with application to RecA. PLoS ONE 10(3):e0116414.
- Track 3: 3D Structure Determination | Track 4: Computational Approaches | Track 5: Structural Molecular Biology
Location:
Session Introduction
Kurt Ballmer-Hofer
Paul Scherrer Institut, Switzerland
Title: Structural analysis of Vascular Endothelial Growth Factor receptors reveals drug-targetable allosteric sites regulating angiogenesis

Biography:
Kurt Ballmer-Hofer focused his research at PSI on the structural and functional analysis of receptor tyrosine kinases, in particular on Vascular Endothelial Growth Factor Receptors, VEGFRs. In collaboration with partner labs his team solved the structures of VEGF ligands, the ligand binding domain of VEGFR-2, and -3, and of the full-length extracellular domain of VEGFR-1 in complex with VEGF. The data of these studies led to the discovery of allosteric receptor regulatory sites in subdomains 4, 5 and 7. Antibodies and DARPins specifically binding to these domains showed strong inhibition of receptor activation and downstream signaling both in vitro and in vivo in angiogenesis model systems.
Abstract:
Vascular Endothelial Growth Factors (VEGFs) regulate blood and lymph vessel development upon activation of three receptor tyrosine kinases (RTKs), VEGFR-1, -2, and -3. Partial structures of VEGFR/VEGF complexes based on single particle electron microscopy, small angle X-ray scattering, and X-ray crystallography revealed the location of VEGF binding and the spatial arrangement of individual receptor subdomains. Here we describe the structure of the full-length VEGFR-1 extracellular domain (ECD) in complex with VEGF-A at 4 Å resolution. We combined X-ray crystallography, single particle electron microscopy, and molecular modeling for structure determination and validation. The structure reveals the molecular details of ligand-induced receptor dimerization, in particular of homotypic receptor interactions in Ig-domains 4, 5, and 7. Functional analyses of ligand binding and receptor activation confirm the relevance of these homotypic contacts for receptor activation and identify them as allosteric regulatory sites of VEGFR-1.
Based on our structural data we also investigated the function of Ig-domains 4, 5 and 7 in VEGFR-2, the primary receptor driving angiogenesis and vasculogenesis in response to VEGF administration. The basic domain structure of VEGFR-2 is very similar to VEGFR-1, the ECD of both receptors consists of 7 Ig-domains, D1-D7. Mutagenesis studies based on the VEGFR-1structure confirmed that Ig-domains 4 and 7 fulfill an essential regulatory function in receptor activation and may thus represent putative targets for pharmacological intervention. We isolated highly specific antibodies and DARPins (Designed Ankyrin Repeat Proteins) specific for domains 4 or 7. A subset of these reagents efficiently blocked receptor activation and inhibited VEGF-dependent signaling in vitro in endothelial cell cultures. Most importantly, a domain 4-specific DARPin efficiently blocked vessel development also in vivo in a mouse angiogenesis model. In this model endothelial cell spheroids were implanted in matrigel into mice, and cell growth and vessel formation were monitored in the absence and presence of inhibitor. Our study thus revealed a novel approach for therapeutic targeting of aberrant blood vessel development.
References:
- Markovic-Mueller, S., Stuttfeld, E., Asthana, M., Weinert, T., Bliven, S., Goldie, K.N., Kisko, K., Capitani, G., and Ballmer-Hofer, K. (2017). Structure of the Full-length VEGFR-1 Extracellular Domain in Complex with VEGF-A. Structure 25, 341-352.
- Leppänen, V.M., Tvorogov, D., Kisko, K., Prota, A.E., Jeltsch, M., Anisimov, A., Markovic-Mueller, S., Stuttfeld, E., Goldie, K.N., Ballmer-Hofer, K., and Alitalo, K. (2013). Structural and mechanistic insights into VEGF receptor 3 ligand binding and activation. Proc. Natl. Acad. Sci. USA 110, 12960-12965.
- Brozzo, M.S., Bjelic, S., Kisko, K., Schleier, T., Leppänen, V.M., Alitalo, K., Winkler, F.K., and Ballmer-Hofer, K. (2012). Thermodynamic and structural description of allosterically regulated VEGF receptor 2 dimerization. Blood 119, 1781-1788.
- Hyde, C.A., Giese, A., Stuttfeld, E., Abram, S.J., Villemagne, D., Schleier, T., Binz, H.K., and Ballmer-Hofer, K. (2012). Targeting the extracellular domains D4 and D7 of VEGFR-2 reveals allosteric receptor regulatory sites. Mol. Cell Biol. 32, 3802-3813.
- Leppänen, V.M., Prota, A.E., Jeltsch, M., Anisimov, A., Kalkkinen, N., Strandin, T., Lankinen, H., Goldman, A., Ballmer-Hofer, K., and Alitalo, K. (2010). Structural determinants of growth factor binding and specificity by VEGF receptor 2. Proc. Natl. Acad. Sci. USA 107, 2425-2430.
Biography:
Jianyong Li is a biochemistry professor at Virginia Tech. He has extensive experience in protein-related studies, including protein expression and purification, protein functional determination and protein structure and function relationships. Particularly worth to mention is the functional establishment of some unique yellow genes in insects and structural and function relationship of enzymes involved in kynurenate synthesis in mammals.
Abstract:
Bacterial effectors are proteins secreted by pathogenic bacteria into host cells through a type 3 or 4 secretion system. These bacterial effectors may help the pathogens to invade host cells and/or suppress its immune system; thereby promoting their infection, survival and reproduction. Effector proteins are primarily responsible for the pathogenicity of a given bacterial pathogen; therefore, learning the specific mechanism but which their effectors enter into host cells may provide insights for disease prevention. Bacterial Type 3 secretion system (T3SS) has been extensively studied. It is a needle-like structure made by a number of structural proteins, which is responsible for transfer of protein effector to host cells. The needle tip is ~ 3 nm, which is smaller than the required dimension for most bacterial effectors. Despite some better understanding of the T3SS structure, how their bacterial effectors gain entry to host cells remains speculative. Using a T3SS-dependant effector as a model from Xanthomonas oryzae (a bacterium causing serious disease to some essential plants), we determined the structures of an effector protein in complex with a chaperone-like protein. In the genome of Xanthomonas oryzae, the coding sequence of effector protein is adjacent to that coding the chaperone-like protein. Our structural analysis indicates that the effector-chaperon complex crystallized as tetramers (1.64 A resolution). The monomer of the protein effector contains a T4 polynucleotide kinase domain, while the monomer of the chaperon includes a novel kinase binding domain. Our data suggest that the chaperone protein interacts with the protein effector in a manner that helps to stabilize the protein effector and prevents the virulence effect of protein effort from harming the bacteria before being transferred to host cells. Currently, efforts are being made to understand the precise roles the chaperon protein plays during the transfer of protein effector to host cells.
References:
- Han et al. (2017) Crystal structure of acetylcholinesterase subunits of the malaria vector Anopheles gambiae. Insect Science (https://www.ncbi.nlm.nih.gov/pubmed/28247978).
- Carlier PR, Bloomquist JR, Totrov M and Li J (2017) Discovery of species-selective and resistance-breaking anticholinesterase insecticide for the malaria mosquito. Current Medical Chemistry (https://www.ncbi.nlm.nih.gov/pubmed/28176636)
- Yang et al. (2016) Kynurenine aminotransferase 3/glutamine transaminase L/cysteine conjugate beta-lyase 2 is a major glutamine transaminase in the mouse kidney. Biochemistry and Biophysics Reports 8: 231-241.
- Han Q et al. (2015) crystal structure of the complex between AvrRxo1-ORF1, a type III effector and its cognate chaperon, AvrRxo1-ORF2. Structure 23: 1900-1909 (http://www.sciencedirect.com/science/article/pii/S0969212615003251).
- Han Q, Robinson H, Ding H, Christensen BM and Li J (2012) Evolution of insect arylalkylamine N-acetyltransferases: structural evidence from the yellow fever mosquito, Aedes aegypti. Proceedings of the National Academy of Sciences 109: 11669-11674.

Biography:
Kyoko Shinzawa-Itoh, associate Professor of University of Hyogo, grew up in Hiroshima Japan. She received her MS degree from Hiroshima University of Graduate School of Integrated Arts and Sciences and her Ph D. in Pharmaceutical Sciences from Hiroshima University of Graduate School of Biomedical & Health Sciences. She worked as assistant professor at the Department of Life Science, Himeji Institute of Technology 1988-2004 and at the Hyogo University of Graduate School of Life Science 2004-2013. She is associate Professor of Picobiology Institute, Graduate School of Life Science University of Hyogo from 2013. She has studied about mitochondrial respiratory complexes.
Abstract:
Mitochondrial cytochrome c oxidase (CcO) transfers electrons from cytochrome c (Cyt.c) to O2 to generate H2O, a process coupled to proton pumping. To elucidate the mechanism of electron transfer, a crystal structure of the complex of CcO and Cyt.c would be invaluable for mechanistic studies. Two-dimensional (2D) crystals of the mammalian Cyt.c–CcO complex were prepared at higher pH (7.4–9.0) with both proteins in the oxidized state (Osuda et al, 2016), but these 2D crystals could not provide us with a structure of sufficient resolution. We optimized 3D crystallization conditions for ferri-Cyt.c and oxidized CcO at high pH and solved the X-ray structure of the complex at 2.0 Å resolution. The specific interaction between Cyt.c and CcO is stabilized by only six electrostatic interactions between side chains within a small contact surface. From a theoretical calculation based on the complex structure, we identified an electron transfer pathway from the heme c of Cyt.c to CuA of CcO via Lys-13 of Cyt.c. Between the two proteins are three water layers, one of which lies between the other two layers without significant direct interaction with either protein. The inter-molecular span between Cyt.c and CcO is longer than those of other complexes by more than 3.0 Å, and the contact surface area of Cyt.c and CcO is smaller than one-third the size of those of other complexes. Cyt.c undergoes large structural fluctuations, using the interacting regions with CcO as a fulcrum. These features of the protein–protein interaction at the docking interface represent the first known example of a new class of inter-protein interaction, which we term “soft and specific”. This interaction is predicted to contribute to the rapid association/dissociation of the Cyt.c–CcO complex, which facilitates the sequential supply of four electrons for the O2 reduction reaction.
References:
- Osuda Y, Shinzawa-Itoh K, Tani K, Maeda S, Yoshikawa S, Tsukihara T, Gerle C (2016) Two-dimensional crystallization of monomeric bovine cytochrome c oxidase with bound cytochrome c in reconstituted lipid membranes. Microscopy. 65(3):263-267.
- Shimada S, Shinzawa-Itoh K, Baba J, Aoe S, Shimada A, Yamashita E, Kang J, Tateno M, Yoshikawa S, Tsukihara T (2017) Complex structure of cytochrome c-cytochrome c oxidase reveals a novel protein-protein interaction mode. EMBO J. 36(3):291-300.
- Shinzawa-Itoh K, Shimomura H, Yanagisawa S, Shimada S, Takahashi R, Oosaki M, Ogura T, TsukiharaT (2016) Purification of active respiratory supercomplex from bovine heart mitochondria enables functional studies. J. Biol. Chem. 291(8):4178-84.
- Hirata K, Shinzawa-Itoh K, Yano N, Takemura S, Kato K, Hatanaka M, Muramoto K, Kawahara T, Tsukihara T, Yamashita E, Tono K, Ueno G, Hikima T, Murakami H, Inubushi Y, Yabashi M, Ishikawa T, Yamamoto M, Ogura T, Sugimoto H, Shen JR, Yoshikawa S, Ago H (2014) Determination of damage-free crystal structure of an X-ray-sensitive protein using an XFEL. Nat Methods. 11(7):734-736.
Jeffrey L Urbauer
The University of Georgia, USA
Title: Oxidative stress, methionine oxidation, and calmodulin structure and function

Biography:
Jeffrey Urbauer earned bachelor’s and doctoral degrees in chemistry from the University of Nebraska-Lincoln. He pursued postdoctoral studies at the University of Wisconsin-Madison as an NIH postdoctoral fellow and at the University of Illinois Urbana-Champaign. He held faculty appointments at the State University of New York at Buffalo, the University of Pennsylvania, and the University of Kansas before joining the faculty in the Department of Chemistry and the Department of Biochemistry and Molecular Biology at the University of Georgia. At the University of Kansas the Mortar Board National College Senior Honor Society awarded him with the Outstanding Educator Award. His research interests include structural biology, protein biophysics and NMR spectroscopy.
Abstract:
Statement of the Problem: Oxidation of methionine residues in proteins to methionine sulfoxide is a prevalent, reversible post-translational modification. Changes in protein structure and function accompany oxidation due to polarity and steric differences between methionine and the sulfoxide. We are investigating the consequences of methionine oxidation in the regulatory protein calmodulin (CaM), a key calcium signal transducer with nine methionine residues, in hydrophobic pockets of its opposing globular domains, which interact with target proteins. CaM with oxidized methionine residues accumulates under conditions of oxidative stress, and because of its central role in biology, it is important to understand the functional effects of these alterations and their physical origins. Methodology: Methionine residues in CaM are easily oxidized in vitro with hydrogen peroxide. To study the effects of oxidation of specific methionine residues, leucine was substituted for methionine at remaining sites. A combination of functional assays, single molecule studies, and NMR spectroscopy were used to assess functional and structural consequences of methionine oxidation. Findings: For the best studied case, activation of the plasma membrane Ca-ATPase (PMCA) by CaM, impaired CaM function is due to oxidation of a single C-terminal methionine. Single molecule experiments indicate non-productive binding of oxidized CaM to the PMCA. High resolution NMR studies demonstrate significant structural perturbation in the C-terminal globular domain of oxidized CaM and an inability to anchor the PMCA to this domain. Conclusion & Significance: The functional effects of methionine oxidation in CaM are highly target dependent, as is the degree to which selective oxidation of particular methionine residues in CaM affects function. The results of CaM activation of the PMCA also indicate that both high-affinity productive and non-productive complexes of oxidized CaM with targets are possible. These facts indicate that a comprehensive understanding of the metabolic consequences of CaM oxidation will be challenging.
References:
- Bartlett RK, Bieber Urbauer RJ, Anbandam A, Smallwood HS, Urbauer JL, Squier TC (2003) Oxidation of Met144 and Met145 in calmodulin blocks calmodulin dependent activation of the plasma membrane ca-ATPase. Biochemistry 42:3231-3238.
- Osborn KD, Bartlett RK, Mandal A, Zaidi A, Bieber Urbauer RJ, Urbauer JL, Galeva N, Williams TD, Johnson CK (2004) Single-molecule dynamics reveal an altered conformation for the autoinhibitory domain of plasma membrane Ca-ATPase bound to oxidatively modified calmodulin. Biochemistry 43:12937-12944.
- Anbanandam A, Bieber Urbauer RJ, Bartlett RK, Smallwood HS, Squier TC, Urbauer, JL (2005) Mediating molecular recognition by methionine oxidation: Conformational switching by oxidation of methionine in the carboxyl-terminal domain of calmodulin. Biochemistry 44:9486-9496.
- Slaughter BD, Bieber Urbauer RJ, Urbauer JL, Johnson CK (2007) Mechanism of calmodulin recognition of the binding domain of isoform 1b of the plasma membrane Ca-ATPase: Kinetic pathway and effects of methionine oxidation. Biochemistry 46: 4045-4054.
- Lubker C, Bieber Urbauer RJ, Moskovitz J, Dove S, Weisemann J, Fedorova M, Urbauer JL, Seifert R (2015) Membranous adenylyl cyclase 1 activation is regulated by oxidation of N- and C-terminal methionine residues in calmodulin. Biochemical Pharmacology 93:196-209.
Miki Senda
High Energy Accelerator Research Organization (KEK), Japan
Title: Molecular mechanism of SHP2 activation by CagA from Helicobacter pylori

Biography:
Miki Senda has completed her PhD at 2008 from Nagaoka University of Technology. She is an assistant professor of Structural Biology Research Center in High Energy Accelerator Research Organization (KEK). She has several collaborations, in which she has worked as an expert of protein crystallization and crystal quality improvement. She received Oxford Cryosystems Low Temperature Prize at the 63rd Annual meeting of the American Crystallographic Association (ACA) in 2013.
Abstract:
Helicobacter pylori, which is known as a major risk factor of stomach cancer, delivers an effector protein CagA into gastric epithelial cells. CagA then promiscuously interact with host proteins, SHP2 and PAR1b, to deregulate these proteins, potentiating oncogenic signaling. CagA comprises an N-terminal structured region and a C-terminal intrinsically disordered region which interacts with the host proteins. We already determined the crystal structure of the N-terminal region of CagA (1–3). The crystal structure revealed that the basic amino-acid cluster in the N-terminal region is utilized to localized CagA at the inner face of the plasma membrane. After localization, short segments including Glu-Pro-Ile-Tyr-Ala (EPIYA) motif in the C-terminal disordered region are phosphorylated by Src and interact with SHP2 to deregulate its phosphatase activity. In this study, we have analyzed the structure-function relationship of the EPIYA-segments of CagA. Based on the sequence flanking each of the EPIYA motifs, four types of EPIYA segments, A, B, C, and D, have been identified (4). It is already known that combinations of the EPIYA segments are geographically different (Western and East Asian CagA) and affect CagA’s oncogenic activity. While Western CagA with EPIYA-A, B, and C segments has weak oncogenic activity, Western CagA with EPIYA-A, B and multiple EPIYA-C segments shows increased oncogenic activity (5). East Asian CagA, which has much higher oncogenic activity than Western ones, typically possesses EPIYA-A, B, and D in the C-terminal region. Our biochemical data revealed that oncogenic activity of CagA is correlated with binding affinity for SH2 domain of SHP2 (SH2_SHP2). We have analyzed the interaction between SH2_SHP2 and the EPIYA-C/D segment using biochemical, crystallographic, and physicochemical methods and revealed two types of activation mechanisms of SHP2. In our presentation, we will report that East Asian and Western CagA utilize two distinct activation mechanisms of SHP2.
References:
- Hayashi T et al., (2012) Tertiary structure-function analysis reveals the pathogenic signaling potentiation mechanism of Helicobacter pylori oncogenic effector CagA. Cell host & microbe 12:20-33.
- Senda M et al., (2016) Use of multiple cryoprotectants to improve diffraction quality from protein crystals. Crystal growth & design 16: 1565-1571.
- Senda M (2016) A comprehensive strategy to obtain high quality crystals. Structural Biology 2016
- Hatakeyama M (2004) Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat. Rev. Cancer 4: 688-694.
- Nagase L et al., (2015) Dramatic increase in SHP2 binding activity of Helicobacter pylori Western CagA by EPIYA-C duplication: its implications in gastric carcinogenesis. Sci. Rep. 5: 15749.
Alfredo De Biasio
Elettra-Sincrotrone Trieste, Italy
Title: Integrative approaches to study the structure and motions of DNA sliding clamps
Time :

Biography:
Alfredo De Biasio’s work focuses on the structure and function of DNA sliding clamps and their complexes operating in DNA replication and repair. He is particularly interested in understanding the mechanisms of sliding of the eukaryotic clamp PCNA, and how these mechanisms are modulated by modifications of the PCNA sliding surface, and the implications in DNA damage avoidance. These problems are tackled by an integrative approach that combines X-ray crystallography, NMR and MD simulations.
Abstract:
Sliding clamps encircle DNA and tether polymerases and other proteins to the genomic template, and are essential factors in DNA replication. Because of the transient interaction that the clamps establish with DNA, the clamp-DNA interface eluded a thorough structural characterization, so that the molecular mechanism for clamp sliding on DNA remained obscure. Here, I will show how the combined use of high-resolution techniques (X-ray crystallography and NMR) and molecular dynamics (MD) simulations allowed to visualize the interactions between the Proliferating Cell Nuclear Antigen (PCNA) – the eukaryotic sliding clamp – and DNA, and to decipher the mechanics of sliding. In addition, recent findings show that the DNA sliding surface of PCNA can be modified to regulate the resistance to DNA damage. From a structural viewpoint, I will reflect on these findings which open a new perspective on PCNA function and offer opportunities to develop tools to manipulate the DNA damage response in cancer treatment.
References:
1. De March, M. et al. Structural basis of human PCNA sliding on DNA. Nat. Commun. 8, 13935 (2017).
2. De Biasio, A. et al. Structure of p15PAF‒PCNA complex and implications for clamp sliding during DNA replication and repair. Nat. Commun. 6, 6439 (2015).

Biography:
Liliane Mouawad was always interested in understanding the mechanism of action of proteins or protein assemblies. This understanding may be based on either molecular simulations or on experiments like NMR. But her expertise is primarily in molecular dynamics simulations and more precisely in normal mode analysis (NMA). She has developed several methods going from the calculation of normal modes of very large systems or of images, to the calculation of the pathway between two protein conformations, to the prediction of the compactness of a calcium-binding protein. Recently she was also involved in docking and virtual screening themes, where she has acquired enough expertise to develop a new consensus methodology to overcome some issues observed in these approaches.
Abstract:
A microtubule is a dynamic system formed of ab-tubulins. The presence of nonhydrolyzable guanosine-5’-triphosphate (GTP)/guanosine diphosphate (GDP) on the b-tubulins provokes microtubule polymerization/depolymerization. Despite the large number of experimental studies of this dynamical process, its mechanism is still unclear. To provide insights into this mechanism, we studied the first depolymerization steps of GDP/GTP-bound microtubules by normal-mode analysis with the all-atom model. We also constructed a depolymerizing microtubule and compared it to cryo-electron microscopy tomograms (cyro-ET). The results show that during depolymerization, the protofilaments not only curve but twist to weaken their lateral interactions. These interactions are stabilized by GTP, but not evenly. Not all of the interface residues are of equal importance: five of them, belonging to the H2-S3 loop, play a special role; acting as a lock whose key is the g-phosphate of GTP. Sequence alignments of several tubulins confirm the importance of these residues.
References:
- Chaput L, Martinez‑Sanz J, Saettel N, Mouawad L (2016) Benchmark of four popular virtual screening programs: construction of the active/decoy dataset remains a major determinant of measured performance. J Cheminform 8:56-72.
- Chaput L, Martinez‑Sanz J, Quiniou E, Rigolet P, Saettel N, Mouawad L (2016) vSDC: a method to improve early recognition in virtual screening when limited experimental resources are available. J Cheminform 8:1-18.
- Quiniou E, Guichard P, Perahia D, Marco S, Mouawad L (2013) An atomistic view of microtubule stabilization by GTP Structure 21: 833–843.
- Martinez-Sanz J, Kateb F, Assairi L, Blouquit Y, Bodenhausen G, Abergel D, Mouawad L, Craescu CT (2010) Structure, dynamics and thermodynamics of the human centrin 2/hSfi1 complex. J. Mol. Biol. 395: 191–204.
- Mouawad L, Isvoran A, Quiniou E, Craescu CT (2009) What determines the degree of compactness of a calcium-binding protein? FEBS Journal 276:1082–1093.
Hyun-Soo Cho
Yonsei University, Korea
Title: Structure and function of a Chloride Pump Rhodopsin from marine bacteria

Biography:
Hyun-Soo Cho’s research aims to understand the structural and functional role of various proteins involved in cancer and immune diseases. He is specialized in X-ray Crystallography to solve protein structures with other biophysical and biochemical techniques including Cryo_EM recently. His ongoing research projects include various enzymes and receptors especially G-Protein Coupled Receptor (GPCR) related with cancer and immune system.
Abstract:
Recently, light-driven sodium pump rhodopsin (NaR/KR2/NDQ rhodopsin) and chloride pump rhodopsin (ClR/NTQ rhodopsin) from marine flavobacteria were identified by metagenomics study. One of them, light-driven sodium pump rhodopsin (NaR) structure was determined. The other one we have solved the first crystal structure of a unique class light-driven chloride pump (ClR) from Nonlabens marinus S1-08, at resolutions of 1.57 Å. Like structured Halorhodopsin (HR), ClR can transfer chloride ion from extracellular to cytosol. Although both ClR and HR are same light-driven chloride pump rhodopsin, we found some evidences that ClR and HR are different in structure and mechanism. The structures reveal two chloride-binding sites, one around the protonated Schiff base and the other on a cytoplasmic loop. We identify a “3 omega motif” formed by three non-consecutive aromatic amino acids that is correlated with the B-C loop orientation. Detailed CIR structural analyses with functional studies in E. coli reveal the chloride ion transduction pathway. Our results help understand the molecular mechanism and physiological role of ClR and provide a structural basis for optogenetic applications.
References:
- Kim K, Kwon SK, Jun SH, Cha JS, Kim H, Lee W, Kim J*, Cho HS*, Crystal structure and functional characterization of a light-driven chloride pump having an NTQ motif. Nature Communications, 7, 12677 (2016)
- Lim Y, Yoo J, Kim MS, Hur M, Lee EH, Hur HS, Lee JC, Lee SN, Park TW, Lee K, Chang KH, Kim K, Kang YJ, Hong KW, Kim SH, Kim YG, Yoon Y, Nam DH, Yang H, Kim DG, Cho HS*, Won J*, GC1118, an Anti-EGFR Antibody with a Distinct Binding Epitope and Superior Inhibitory Activity against High-Affinity EGFR Ligands. Molecular Cancer Therapeutics, 15, 251-263 (2016)
- Lee J, Choi HJ, Yun M, Kang YJ, Jung JE, Ryu Y, Kim TY, Cha YJ, Cho HS*, Min JJ*, Chung CW*, Kim HS*, Enzymatic Prenylation and Oxime Ligation for the Synthesis of Stable and Homogeneous Protein-Drug Conjugates for Targeted Therapy. ANGEWANDTE CHEMIE-INTERNATIONAL EDITION, 54, 12020-12024 (2015)
- Jeong SA, Kim K, Lee JH, Cha JS, Khadka P, Cho HS*, Chung IK*, Akt-mediated phosphorylation increases the binding affinity of hTERT for importin α to promote nuclear translocation. Journal of Cell Science, 128, 2287-2301 (2015)
- Cho YS, Yoo J, Park S, Cho HS*, The structures of the kinase domain and UBA domain of MPK38 suggest the activation mechanism for kinase activity. Acta Crystallography D, 70, 514-521 (2014)
Stanislav Engel
Ben-Gurion University, Israel
Title: Construction of Structural Mimetics of the Thyrotropin Receptor Intracellular Domain

Biography:
Stanislav Engel, Ph.D., now is an Assistant Professor in the Department of Clinical Biochemistry and Pharmacology, Faculty of Health Sciences, The National Institute for Biotechnology, Ben-Gurion University in the Negev, Beer-Sheva, Israel. He got his B Sc in biochemistry, M Sc and Ph.D. in biochemistry and biotechnology engineering at the Ben-Gurion University in the Negev. Currently, Dr. Stanislav Engel’ researches focus on the understanding the structural basis of “protein misfolding” diseases, such as ALS, and structure-based drug discovery.
Abstract:
Dissecting G protein-coupled receptors (GPCR) signaling in terms of the pathways being activated will boost our understanding of the molecular fundamentals of hormone action. The structural determinants governing the selectivity of GPCR/G protein coupling, however, remain obscure. The selectivity of GPCR/G protein recognition appears to be determined by both specific inter-residue interactions and features related to the overall 3D conformation of the ICD. It appears, therefore, that to elucidate the fundamentals of the selectivity of GPCR/G protein recognition, a comprehensive analysis of the structure-activity relationships of multiple GPCR complexes with different G protein isoforms is required. However, enormous technical difficulties associated with the isolation of functional receptors in quantities required for direct structural studies effectively impede progress in the field. Methodology: We constructed the functional mimetics of the intracellular domain (ICD) of a model GPCR - thyrotropin receptor (TSHR), based on a unique scaffold, 6-Helix, an artificial protein that was derived from the elements of the trimer-of-hairpins structure of HIV gp41 and represents a bundle of six a-helices. Findings: The 6-Helix scaffold, which endowed the substituted TSHR ICD elements with spatial constraints analogous to those, found in native receptors, enabled the reconstitution of a microdomain comprising the intracellular loops ICL-2 and ICL-3, which is capable of binding and activating Ga-(s). Conclusion & Significance: By using a soluble scaffold, which furnishes peptides derived from the GPCR ICD with spatial constraints similar to those, found in native receptors, the reconstitution of a native-like G protein-recognition epitope can be facilitated. The 6-Helix-based mimetics could be used as a platform to study the molecular basis of GPCR/G protein recognition. Such knowledge could lead to the development of novel therapeutic strategies for GPCR-related disorders by targeting the GPCR/G protein interfaces and help counteract cellular dysfunctions via focused tuning of GPCR signaling.
References:
- Y. Y. Kuttner and S. Engel, Protein hot spots - the islands of stability, Journal of Molecular Biology, 415(2):419-28 (2012)
- Y. Y. Kuttner, T. Nagar and S. Engel, Surface dynamics in allosteric regulation of protein-protein interactions: Modulation of calmodulin functions by Ca2+, PLOS Computational Biology, 9(4): e1003028 (2013)
- B. Katzman, M. Vyazmensky, O. Press, M. Volokita and S. Engel, Tethered ribozyme ligation enables detection of molecular proximity in homogeneous solutions, Biotechnology Journal, 10 (3):379–385 (2015)
- A. Kuzmina, K. Vaknin, G. Gdalevsky, M. Vyazmensky, R.S. Marks, R. Taube and S. Engel, Functional Mimetics of the HIV-1 CCR5 Co-Receptor Displayed on the Surface of Magnetic Liposomes, PLOS One, 10(12): e01440432015 (2015)
- R. Osman, M. Mezei and S. Engel, The role of protein “Stability patches” in molecular recognition: A case study of the human growth hormone-receptor complex, Journal of Computational Chemistry, 37 (10):913-919 (2016)
Mi Sun Jin
GIST, Republic of Korea
Title: Structural Basis of Sodium/Citrate Symporter as a Secondary Transporter

Biography:
Mi Sun Jin - Assistant Professor, 2014-Present, School of Life Sciences, GIST;
Education - B.S. 2002, Sogang University; M.S. 2004, KAIST; Ph.D. 2008, KAIST (advisor: Jie-Oh Lee)
Professional Experience - Postdoctoral Fellow, 2008-2009, KAIST (advisor: Jie-Oh Lee),
Postdoctoral Fellow, 2009-2013, Purdue University (advisor: Jue Chen),
Research Specialist, 2013-2014, Purdue University
Abstract:
The sodium-dependent citrate transporter of Klebsiella pneumoniae (KpCitS) belongs to the 2-hydroxycarboxylate transporter (2-HCT) family and allows the cell to use citrate as sole carbon and energy source in anaerobic conditions. We present crystal structures of KpCitS in its citrate-bound outward-facing as well as citrate-free inward-facing state. The structure of the asymmetric KpCitS homodimer containing both outward- and inward-open protomers was also determined. The structures reveal that the KpCitS dimerization domain remains stationary throughout the transport cycle due to an extensive hydrogen bond network as well as hydrophobic interactions. In contrast, its transport domain undergoes a ~35° rigid-body rotation and a ~17 Å translocation perpendicular to the membrane to expose the substrate-binding site alternately to either side of the membrane. Homology models of two other 2-HCT proteins based on the KpCitS structure offer structural insights into their differences in substrate specificity at a molecular level. On the basis of our results and previous biochemical data, we propose that the activity of the 2-HCT family of transporters involves an elevator-like movement in which the transport domain itself traverses the lipid bilayer, carrying the substrate into the cell in a sodium-dependent manner.
References:
- Kim JH, Song DH, Youn SJ, Kim JW, Cho G, Kim SC, Lee H, Jin MS, Lee JO (2016) Crystal structures of mono- and bi-specific diabodies and reduction of their structural flexibility by introduction of disulfide bridges at the Fv interface. Scientific reports, 6, 34515.
- Mi Sun Jin, Michael L. Oldham, Qiuju Zhang, Jue Chen (2012) Crystal structure of the multidrug transporter P-glycoprotein from C. elegans. Nature, 490, 566-9.
- Jin Young Kang, Xuehua Nan, Mi Sun Jin, Suk-Jun Youn, Young Hee Ryu, Shinji Ma, Seung Hyun Han, Sang-Gi Paik, Hayyoung Lee, Jie-Oh Lee (2009) Recognition of lipopeptide patterns by TLR2-TLR6 heterodimer. Immunity, 31, 873-84.
- Mi Sun Jin, Jie-Oh Lee (2008) Structures of the Toll-like Receptor Family and Its Ligand Complexes. Immunity, 29, 182-91.
- Mi Sun Jin, Sung Eun Kim, Jin Young Heo, Mi Eun Lee, Ho Min Kim, Sang-Gi Paik, Hayyoung Lee, Jie-Oh Lee (2007) Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a triacylated lipopeptide. Cell, 130, 1071-82.
Pedro J. de Pablo
Universidad Autonoma de Madrid, Spain.
Title: Atomic Force Microscopy of virus capsids uncover the interplay between mechanics, structure and function

Biography:
Pedro J. de Pablo has his expertise in the application of Atomic Force Microscopy to the investigation of individual viruses. He studied Condensed Matter Physics at the Universidad Autonoma de Madrid and worked with a variety of molecular wires, including carbon nanotubes and DNA during his PhD. Then he moved for a postoc in the Vrije Universiteit to apply AFM to the study of molecular motors (kinesin) and viruses. He stablished his own group back in Madrid, where during the last 10 years has been applying AFM to unveil the structure-function interplay of viruses by measuring thir physical properties, such as mechanics and electrostatics.
Abstract:
The basic architecture of a virus consists of the capsid, a shell made up of repeating protein subunits, which packs, shuttles and delivers their genome at the right place and moment. Viral particles are endorsed with specific physicochemical properties, which confer to their structures certain meta-stability whose modulation permits fulfilling each task of the viral cycle. These natural designed capabilities have impelled using viral capsids as protein containers of artificial cargoes (drugs, polymers, enzymes, minerals) with applications in biomedical and materials sciences. Both natural and artificial protein cages (1) have to protect their cargo against a variety of physicochemical aggressive environments, including molecular impacts of highly crowded media, thermal and chemical stresses, and osmotic shocks. Viral cages stability under these ambiences depend not only on the ultimate structure of the external capsid, which rely on the interactions between protein subunits, but also on the nature of the cargo. During the last decade our lab has focused on the study of protein cages with Atomic Force Microscopy (AFM) (figure 1). We are interested in stablishing links of their mechanical properties with their structure and function. In particular, mechanics provide information about the cargo storage strategies of both natural and virus-derived protein cages (2,3). Mechanical fatigue has revealed as a nanosurgery tool to unveil the strength of the capisd subunit bonds (4). We also interrogated the electrostatics of individual protein shells (5). Our AFM-fluorescence combination provided information about DNA diffusing out cracked-open protein cages in real time (6).
References:
- Llauró et al. Nanoscale, 2016, 8, 9328.
- Hernando-Pérez et al. Small, 2012, 8, 2336.
- Ortega-Esteban et al. ACS Nano, 2015, 9, 10826.
- Hernando-Pérez et al. Nature Communications, 2014, 5, 4520.
- Hernando-Pérez et al. Nanoscale, 2015, 7, 17289.

Biography:
The Kolaczkowski Lab uses a combination of computational biology, statistics, structural modeling and biochemistry to examine how molecular systems evolve. We are particularly interested in the evolution of innate immunity in animals and plants. Dr. Kolaczkowski’s research program focuses on developing cutting-edge tools for the characterization of molecular-evolutionary processes and applying these approaches to learn how long-term protein evolution works. Dr. Kolaczkowski’s recent work has advanced the fields of ancestral sequence resurrection (ASR) and protein function prediction while shedding new light on the evolution of innate antiviral immunity and RNA interference.
Abstract:
- Pugh C, Kolaczkowski O, Manny A, Korithoski B, Kolaczkowski B (2016) Resurrecting the ancestral structural dynamics of an antiviral immune receptor: Adaptive binding pocket reorganization repeatedly shifts RNA preference. BMC Evolutionary Biology 16(1):241.
- Dias R, Kolaczkowski B (2015) Different combinations of atomic interactions predict protein-small molecule and protein-DNA/RNA affinities with similar accuracy. Proteins 83(11):2100-2114.
- Korithoski B, Kolaczkowski O, Mukherjee K, Kola R, Earl C, Kolaczkowski B (2015) Evolution of a novel antiviral immune signaling interaction by partial-gene duplication. PLoS One 10(9):e0137276.
- Mukherjee K, Korithoski B, Kolaczkowski B (2014) Ancient origins of vertebrate-specific innate antiviral immunity. Molecular Biology and Evolution 31(1):140-153.
- Mukherjee K, Campos H, Kolaczkowski B (2013) Evolution of animal and plant Dicers: Early parallel duplications and recurrent adaptation of antiviral RNA binding in plants. Molecular Biology and Evolution 30(3):627-641.
John van Noort
Leiden University, The Netherlands
Title: The structure of chromatin; single-molecule experiments on model fibers and real genes

Biography:
Chromatin is the ubiquitous protein-DNA complex that forms the structural basis of DNA condensation in all eukaryotic organisms. Packaging and depackaging of chromatin, called chromatin remodeling, plays a central role in all cellular processes that involve chromosomes such as transcription, replication, recombination and repair. Detailed knowledge of the principles and mechanisms underlying this control of DNA condensation is thus vital for understanding many diseases, including neurological disorders and cancer. The physical mechanisms governing these processes however, are still largely unknown. I am interested in developing and using modern biophysical techniques to unravel the physics behind DNA condensation and its role in transcription regulation.
Abstract:
- Multiplexing genetic and nucleosome positioning codes: a computational approach (2016) B Eslami-Mossallam, RD Schram, M Tompitak, J van Noort, H Schiessel PloS one 11 (6), e0156905
- Quantitative analysis of single-molecule force spectroscopy on folded chromatin fibers (2016) H Meng, K Andresen, J Van Noort Nucleic acids research 43 (7), 3578-3590
- spFRET reveals changes in nucleosome breathing by neighboring nucleosomes (2015) R Buning, W Kropff, K Martens, J van Noort Journal of Physics: Condensed Matter 27 (6), 064103
- Histone H3 phosphorylation near the nucleosome dyad alters chromatin structure (2014) Justin A North, Marek Šimon, Michelle B Ferdinand, Matthew A Shoffner, Jonathan W Picking, Cecil J Howard, Alex M Mooney, John van Noort, Michael G Poirier, Jennifer J Ottesen Nucleic acids research 42 (8), 4922-4933
- Sequence-based prediction of single nucleosome positioning and genome-wide nucleosome occupancy (2012) T van der Heijden, JJFA van Vugt, C Logie, J van Noort Proceedings of the National Academy of Sciences 109 (38), E2514-E25225.
- Track 6: Frontiers in Structural Biology
Session Introduction
Petra Fromme
Arizona State University, USA
Title: Dynamics of Biomolecules “In Action†Studied with X-ray Free Electron Lasers

Biography:
Petra Fromme received her masters at the Free University in Berlin in Biochemistry (1985) and then received her doctorate in Chemistry at the Technical University in Berlin (1988) where she then became a professor in 1992. During this time she developed and pursued her fascination with understanding the function of membrane proteins by investigating and determining their atomic structures. In 2002, Dr. Fromme joined Arizona State University as a professor of Chemistry and Biochemsitry where she has worked with distinguished colleagues from around the world to pioneer a new technique for imaging proteins using extraordinarily powerful x-ray lasers that has the capability to make movies of these fascinating proteins in action.
Abstract:
Biomolecules are highly dynamic; however, most structures only provide a static picture of the molecule. Serial Femtosecond Crystallography (SFX) provides a novel concept for structure determination, where X-ray diffraction “snapshots” are collected from a fully hydrated stream of nanocrystals, using femtosecond pulses from high energy X-ray free-electron lasers (XFELs), where diffraction is observed before destruction takes effect [1]. The first proof of concept of serial femtosecond crystallography was achieved using Photosystem I, a larger membrane protein complex involved in Photosynthesis as a model system [2]. The structure of non-damaged biomolecules can now be determined, unraveling their function at the atomic scale [3],[4] that include important human membrane-bound receptors [6], [8] [9]. SFX opens a new avenue for determination of protein dynamics, with the goal of molecular movies of biomolecules “in action”. First experiments on the proof of principle for time resolved serial femtosecond nanocrystallography have been performed on proteins in Photosynthesis, where first snapshots of steps in water splitting reaction have been observed [10]. A new concept based on continuous X-ray diffraction extends resolution beyond Bragg diffraction and allows for direct phasing of X-ray diffraction data [11]. TR-SFX studies extend to atomic resolution where the first steps in photosensing were recently revealed at a time scale of femtoseconds using the photoactive yellow protein [12,13]. This pioneering work paves the way for the determination of molecular movies of the dynamics of membrane proteins "at work" in the future.
The talk will close with a progress report on the development of compact femto and attosecond X-ray Sources at DESY (AXSIS) and at ASU (CXLS and CXFEL) , which will provide unique new opportunities to study the ultrafast dynamics of reactions in photosynthesis with a combination of X-ray diffraction, X-ray spectroscopy and ultrafast optical spectroscopy [14].
References:
- Barty A, Caleman C, Aquila A, Timneanu N, Lomb L, et al. (2012) Self-terminating diffraction gates femtosecond X-ray nanocrystallography measurements. Nature Photonics 6: 35-40.
- Chapman HN, Fromme P, Barty A, White TA, Kirian RA, et al. (2011) Femtosecond X-ray protein nanocrystallography. Nature 470: 73-U81.
- Boutet S, Lomb L, Williams GJ, Barends TRM, Aquila A, et al. (2012) High-Resolution Protein Structure Determination by Serial Femtosecond Crystallography. Science 337: 362-364.
- Redecke L, Nass K, DePonte DP, White TA, Rehders D, et al. (2013) Natively Inhibited Trypanosoma brucei Cathepsin B Structure Determined by Using an X-ray Laser. Science 339: 227-230.
- Kang YY, Zhou XE, Gao X, He YZ, Liu W, et al. (2015) Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature 523: 561-+.
Maria Bykhovskaia
Wayne State University, USA
Title: Protein Machinery Regulating the Synaptic Vesicle Fusion

Biography:
Maria Bykhovskaia is an expert in synaptic transmission. Her lab combines molecular modeling and computations with electrophysiology, microscopy, and molecular biology approaches. She hold a Professor’s position in the WSU. Her Ph.D. training was in protein molecular modeling, and subsequently she used a postdoc in computational neuroscience to initiate a career devoted to the study of presynaptic mechanisms and plasticity. As a PI, she has developed in her lab expertise in electrophysiology, live confocal imaging, and electron microscopy. The lab combines these experimental approaches with mathematical modeling to understand the fundamental mechanisms of release of neuronal transmitters.
Abstract:
Neuronal transmitters are released via the fusion of synaptic vesicles with the plasma membrane. Vesicles dock to the membrane via a protein complex termed SNARE, which contains membrane attached (t-SNARE) and vesicle attached (v-SNARE) proteins. The fusion occurs in response to a calcium inflow, and the vesicle protein Synaptotagmin (Syt) serves as a calcium sensor. A cytosolic protein Complexin (Cpx) interacts with the SNARE complex, restricting the spontaneous fusion. Although molecular interactions of these proteins have been extensively studied, it is still debated how Syt dynamically interacts with the SNARE protein complex, Cpx, and lipid bilayers to trigger lipid merging. To elucidate these mechanisms, we combined molecular dynamics (MD) simulations with molecular biology and genetic approaches in Drosophila. Basing on MD simulations, we created a model of the protein fusion machinery wherein Cpx dynamically interacts with v-SNARE, preventing full SNARE assembly. Our MD simulations also elucidated how Syt interacts with lipid bilayers, causing lipid bulging that may precede the formation of the stalk and the fusion pore opening. Finally, our simulations predicted direct interactions of Syt with the SNARE-bound Cpx. The developed molecular model enabled us to predict new mutations in v-SNARE and Cpx that alter the fusion process. To test these predictions, we generated Drosophila lines with single point mutations and investigated how these mutations affect the kinetics of transmitter release. The results of these experiments suggest that our model creates the basis for systematic approach to manipulating the fusion machinery based on theoretical predictions derived from MD simulations.
References:
- Feliciano P., H. Matos, R. Andrade, and M. Bykhovskaia. 2017. Synapsin II regulation of GABAergic synaptic transmission is dependent on interneuron subtype. J. Neurosci. doi: 10.1523/JNEUROSCI.0844-16.2016.
- Sabeva N., Cho. R., Vasin, A. Gonzalez, J.T. Littleton, and M. Bykhovskaia. (2016) Complexin Mutants Reveal Partial Segregation Between Recycling Pathways that Drive Evoked and Spontaneous Neurotransmission J Neurosci 3:383-396.
- Vasin, A., Volfson, D., Littleton, J. T., and Bykhovskaia, M. (2016) Interaction of the Complexin Accessory Helix with Synaptobrevin Regulates Spontaneous Fusion, Biophys J 111, 1954-1964.
- Byhhovskaia M. 2015. Calcium binding promotes conformation flexibility of neuronal calcium sensor Synaptotagmin 1. Biophys. J. 108:22507-20.
- Fourtoul, N., Singh P., Hui C.Y., Bykhovskaia M., and A. Jagota. 2015. Coarse-Grained Model of the Snare Complex Determines the Number of Snares Required for Docking. Biophys. J. 108:2258-69.
Senyon Teddy Choe
University of California San Diego, USA
Title: Designer Biologics: BMP Chimeras and their clinical potential

Biography:
Senyon Teddy Choe, is a Professor of Biology and the founding director of Drug Discovery Collaboratory at UCSD. After his Ph.D study in Biophysics and Medical Physics at Univ. California, Berkeley. He joined the Salk Institute in 1993 as the founding faculty member of the Institute’s new Structural Biology Laboratory, and remained so through 2015. His research group has focused on understanding how cells talk to each other. An extension of these works explores designing synthetic biologics to modulate stem cells and sick cells directly. His major honors include election in 1999 to the Fellow of American Association for the Advancement of Science. He recently founded joint Center for Biosciences to translate discovery to medical applications to focus on protein engineering and developing new stem cell therapy. He currently leads Mogam Institute for Biomedical Research in Korea aiming for biologics discovery in the areas of infectious diseases and cancer.
Abstract:
Discovery of new biologics presents new challenges and opportunities and revenues for biologics are rapidly growing in biopharmaceutical industry. We exploited our detailed structural knowledge of three-dimensional structures of BMPs, their receptors, and their antagonists to engineer synthetic BMP ligands. By use of a novel protein engineering strategy that we termed RASCH (Random Assembly of Segmental Chimera and Heteromers), we have set out to design a synthetic biologics (synbiologics®) for bone and cartilage therapy. In the case of bone fusion, we have used Activin and BMP-2, which are the members of TGF-beta superfamily, to create AB204 (Allendorph et al., 2011). AB204 is a synthetic 50:50 chimera of the two ligands, which indeed shows highly effective bone-forming capability (Yoon et al., 2014). In the case of cartilage, we have used Activin and BMP-6 to create AB604, again a 50:50 chimera of the two. AB604 shows all the properties of super BMP6, surpassing the functional characteristics of natural BMP6.
This approach promises to be a very powerful way to harness different biological functionalities of natural ligands to merge into one synthetic designer molecule such that it goes beyond what Mother Nature could provide a new means to meet various unmet clinical needs.
References:
- Allendorph, G., Read, J.D., Kawakami, Y., Kelber, J.A., Isaacs, M.J., Choe, S. (2011) Designer TGF-beta superfamily ligands with diversified functionality. PLoS One, 6, e26402.
- Kwiatkowski, W., Gray, P.C., Choe, S. (2014) Engineering TGF-beta superfamily ligands for clinical applications. Trends Pharmacol. Sci., 35:648-57.
- Yoon, B., Esquivies, L., Ahn, C., Gray, P. C., Ye, S.-K., Kwiatkowski, W., Choe, S. (2014) An Activin A/BMP2 chimera displays osteogenic activity superior to that of BMP2. J. Bone and Mineral Research. Online April 1, 29:1950-9
- Yoon, B., Lee, J.H., Na, K., Cho, J., Choe,S. (2015) The toxicological evaluation of repetitive 2- and 4-week intravenous injection of Activin A/BMP-2 chimera (AB204) into rats. Regulatory Toxicology and Pharmacology, 73, 1-8.
- Yoon, B., Lee, J.H., Na, K., Ahn, C., Cho, J., Lee, S.-H., Ahn, H.C., Choi, J., Oh, H., Kim, B.M., Choe, S. (2016) The effects of a single intravenous injection of novel Activin A/BMP-2 chimera (AB204) on toxicity and the respiratory and central nervous systems. Drug and Chemical Toxicology, 39(3):284-9.
John B. Bruning
The University of Adelaide, Australia
Title: Structural mechanism of partial agonists and antagonists of PPARgamma for use as antidiabetics

Biography:
I received by BSc from Texas A&M University in 1997. I began crystallography in the laboratory of Yousif Shamoo at Rice University. During my graduate studies I worked on the structural mechanism of the human sliding clamp and its interactions with DNA replication proteins. I received my PhD in 2005. I completed 2 successful post-docs. The first was at the Scripps Research Institute from 2005-2007 working on structural studies of nuclear receptors including PPAR, RXR, ER, and TR. My second post-doc was with Jim Sacchettini in the Houston Medical centre and I was a part of the TB structural genomics consortium. I received my first faculty position at the University of Adelaide in 2012 as a lecturer. I was tenured in 2015 and promoted to Senior Lecturer in 2016. Do to my continued collaboration with Scripps, I was also appointed adjunct professor of the Scripps Research Institute in 2016.
Abstract:
Synthetic full agonists of PPARγ have been prescribed for the treatment of diabetes due to their ability to regulate glucose homeostasis and insulin sensitization. While the use of full agonists of PPARγ has been hampered due to severe side effects, partial agonists and antagonists have shown promise due to their decreased incidence of such side effects in preclinical models. No kinetic information has been forthcoming in regard to the mechanism of full versus partial agonism of PPARγ to date and little structural and dynamic information is available which can shed light on the mechanistic difference between full and partial agonists as well as antagonists. We have used X-ray crystallography, cellular assays, Hydrogen Deuterium Exchange (HDX), and Surface Plasmon Resonance (SPR) to probe the mechanism of several PPARγ partial agonists and antagonists. Our findings demonstrate that not only do partial agonists and antagonists act through distinct transcriptional mechanisms, they also demonstrate differences in structure, dynamics, and kinetics as compared to full agonists.
References:
- “X-ray Crystal Structure of Rivoglitazone bound to PPARγ and PPAR Subtype Selectivity of TZDs.” Rajapaksha H, Bhatia H, Wegener K, Petrovsky N, Bruning JB. Biochim Biophys Acta. (2017 May 9).
- “Structure-Activity Relationship of 2,4-Dichloro-N-(3,5-dichloro-4-(quinolin-3-yloxy)phenyl)benzenesulfonamide (INT131) Analogs for PPARγ-Targeted Antidiabetics.” Frkic RL, He Y, Rodriguez BB, Chang MR, Kuruvilla D, Ciesla A, Abell AD, Kamenecka TM, Griffin PR, Bruning JB. J Med Chem. (2017 May 22).
- “PPARG Post-translational Modifications Regulate Bone Formation and Bone Resorption.” Stechschulte LA, Czernik PJ, Rotter ZC, Tausif FN, Corzo CA, Marciano DP, Asteian A, Zheng J, Bruning JB, Kamenecka TM, Rosen CJ, Griffin PR, Lecka-Czernik B. EBioMedicine. (2016 Aug;10:174-84).
- “SR2067 Reveals a Unique Kinetic and Structural Signature for PPARγ Partial Agonism.” van Marrewijk LM, Polyak SW, Hijnen M, Kuruvilla D, Chang MR, Shin Y, Kamenecka TM, Griffin PR, Bruning JB. ACS Chem Biol. (2016 Jan 15);11(1):273-83.
- “Structural mechanism for signal transduction in RXR nuclear receptor heterodimers.” Kojetin DJ, Matta-Camacho E, Hughes TS, Srinivasan S, Nwachukwu JC, Cavett V, Nowak J, Chalmers MJ, Marciano DP, Kamenecka TM, Shulman AI, Rance M, Griffin PR, Bruning JB, Nettles KW. Nat Commun. (2015 Aug 20);6:8013.
Luba Tchertanov
CMLA ENS Cachan, France
Title: How Conformational Dynamics Descriptors may help in remodeling of allosteric Regulation in Proteins
Biography:
Luba Tchertanov, Research Director at CNRS-France, leader of the Bioinformatics, Molecular Dynamics and Modeling (BiMoDyM) team in Centre Mathématiques et leurs Applications (CMLA-CNRS) at the Ecole Normale Supérieure (ENS) de Cachan. She has multidisciplinary high-level skills, with extensive experience in structural biology, molecular modelling and numerical simulation (more than 100 papers in peer-reviewed journals). She coordinated or contributed as team-leader to different research projects (CEE, ANR, Fondation de France, OSEO, SIDACTION) and industrial contracts (LIPHATECH, the SERVIER Institute, the Pierre FABRE Laboratory, UNILIVER).
The research topics are focused on exploration of protein structure–dynamics–function relations. In particular, L. Tchertanov is working at the mechanisms of the receptors activation, the mechanisms of resistance to inhibitors, the conformational plasticity and dynamics of inter-molecular interactions and molecular recognition. She is most specifically interested in description of allosteric regulation at an atomistic level. Important part of research is dedicated to the development of new methodology and computing tools for description of proteins dynamics.
Abstract:
Allostery controls nearly all biological processes, and it has been declared Monod to be “the second secrete of life” after the genome. This universal phenomenon in nature represents a target response on a perturbation (e.g. a ligand binding) leading to a functional change at the target through alteration of the structure or dynamics. Such an event can be described in terms of a large-scale transmission of information between residues. This concept is the cornerstone of our method MONETA that delivers descriptor encoding of the communication network in a protein [1]. Using MONETA, we described the allosteric regulation of several proteins involved in cell signalling. Studying the receptors tyrosine kinases (RTKs), KIT and CSF-1R, and their numerous clinically-relevant mutants, we showed that the allosteric communications between the major regulating fragments in the native proteins were disrupted by the gain-of-function mutations [2-4]. The diverging impact of equivalent mutations on communication in homologue RTKs permits us to distinguish between the mutation-induced effects that lead to the constitutive activation of KIT and the mutation-induced effects promoted the resistance in CSF-1R. In STAT5s, RTK downstream signalling proteins, we showed the sequence-dependent asymmetry in the STAT5s’ communications and their different responses to phosphorylation [5]. Our recent study provided a fascinating illustration of how the binding of agonist ligands controls intrinsic conformational dynamics in human NMDA receptors that stabilize the channel opening. The allosteric binding sites, which were identified by a pockets search at the proteins surface adjacent to the communication pathway (Figure 1), may constitute valid targets for the development of inhibitors able to modulate the function-related communication properties of a protein. Such communication-inspired and communication-targeted modulation may selectively block several activation or post-transduction processes. Our work opens the way to novel and rational strategies for the definition of targets, and the development of efficient target-specific inhibitors.
References:
- Allain A, Chauvot de Beauchêne I, Langenfeld F, Guarracino Y, Laine E, Tchertanov L. (2014). Allosteric Pathway Identification through Network Analysis from Molecular Dynamics Simulations to Interactive 2D and 3D Graphs. Faraday Disc., 169, 1-18.
- Chauvot de Beauchêne I, Alain A, Panel N, Laine E, Trouvé A, Dubreuil P. and Tchertanov L. (2014). Oncogenic mutations of KIT receptor differentially modulate tyrosine kinase activity and drug susceptibility. PLOS Comput. Biol.;10(7):e1003749.
- Da Silva Figueiredo Celestino Gomes P, Chauvot de Beauchêne I, Panel N, Pascutti P, Solary E, Tchertanov L. Insight on Mutation-Induced Resistance from Molecular Dynamics Simulations of Wild-type and mutated CSF-1R and KIT. (2016) PLoS ONE.11(7):e0160165.
- Chauvot de Beauchêne I, Tchertanov L. (2016). How Missense Mutations in Receptors Tyrosine Kinases impact Constitutive Activity and alternate Drug Sensitivity: Insights from Molecular Dynamics Simulations. Invited Research highlight for Receptors & Clinical Investigation. Vol.3, e1372.
- Langenfeld F, Guarracino Y, Arock M, Trouvé A,Tchertanov L. (2015). How intrinsic molecular dynamics controls intramolecular communication in Signal Transducers and Activators of Transcription Factor STAT5. PLOS ONE. Dec 30;10(12):e0145142.
Sangwook Wu
Pukyong National University, South Korea
Title: Study on the conformational transition between the alternative and collapsed form of prethrombin-2: targeted molecular dynamics and free energy sampling
Biography:
Sangwook Wu received his B.A. degree in Biochemistry from Yonsei University (Korea) in 1990. After working as a Scientist at Samsung Display Devices (1995-1999), he obtained his Ph.D. in theoretical condensed matter physics from Iowa State University (1999-2005). He joined the computational biophysics lab at UNC-Chapel Hill (Dr. Lee Pedersen) as postdoctoral research associate (2005-2014). From 2014 to the present, he has been a faculty member at Pukyong National University in Korea. His research interests are in the areas of computational dynamics of biological macromolecules.
Abstract:
The alternative and collapsed forms of prethrombin-2 are revealed by X-ray crystallogrphy. We analyzed the conformational transition from the alternative to the collapsed form employing targeted molecular dynamics simulation and 2-dimensional free energy landscape using WHAM method. Some hydrophobic residues (W60d, W148, W215, and F227) show a significant difference between the two conformations in the conformational transition process. We show that the four hydrophobic residues undergo concerted movement from dimer to trimer transition via tetramer state in the conformational change from the alternative to the collapsed form. Also, we reveal that the concerted movement of the four hydrophobic residues is controlled by movement of specific loop regions behind. In this study, we discuss the difference between the transition path generated by the targeted Mplease let m eD simulation and the transition path with minimum Boltzmann weighting on the two-dimensional free energy surface (FES).
References:
- S. Wu (2016) Loop-driven conformational transition between the alternative and collapsed form of prethrombin-2: Targeted Molecular Dynamics study, Journal of Biomolecular Structure and Dynamics, DOI:10.1080/07391102.2015.1134347.
- S. Wu, C. J. Lee, and L. G. Pedersen (2014) Analysis on long-range residue–residue communication using molecular dynamics, Proteins: Structure, Function and Bioinformatics, 82: 2896 .
- S. Wu, W. A. Beard, L. G. Pedersen, and S. Wilson (2014) Structural comparison of DNA polymerase architecture suggest a nucleotide gateway to the polymerase active site”, Chemical Review, 114: 2759.
- S. Wu, J. Tie, D. W. Stafford, and L. G. Pedersen (2014) Membrane topology for human vitamin K epoxide reductase (VKOR), J Thromb Haemost, 12:112.
- S. Wu, S. Liu, S. Sim, L. G. Pedersen (2012) Weak antiferromagnetic coupling via a superexchange interaction between Mn (II)–Mn (II) ions: A QM/MM study of the active site of human cytosolic X-propyl aminopeptidase P”, Journal of Physical Chemistry Letter, 3: 2293.
Ramakrishna Vadrevu
Birla Institute of Technology and Science, India
Title: Structural and Molecular Dynamics Analysis of the super secondary motifs from TIM barrel proteins: Implications for folding and engineering of foldable building blocks for the assembly of TIMs

Biography:
Ramakrishna Vadrevu received his Masters degree in Physical Chemistry and his Ph.D. degree in Biophysical Chemistry. He spent few years at the Pennyslvania State University and later at University of Massachusetts Medical School as a post doctoral fellow with Prof. C. Robert Mathews. Since 2008 he has been a faculty at the Birla Institute of Technology and Science-Pilani, Hyderabad Campus in the department of Biological Sciences.
His research focuses on understanding the role cellular environment on protein stability and folding. His research interests also include protein design and engineering, amyloid material and its applications.
Abstract:
Statement of the Problem: The acquired complex three-dimensional structure of proteins is a culmination of simple structural fragments like a-a, b-b, a-b and b-a units. Thus, tertiary structures can be seen as a combination of basic building block motifs implying that all complex protein structures have evolved from the assembly of small independently folding super secondary structures. The TIM barrel proteins are made up of a regular repeating bab motif resulting in the strands and helices in an alternating repetitive pattern. Experimental and theoretical studies have revealed that bab unit acts as a minimal unit of stability. The success of designing super secondary motifs that fold in isolation underscores the prospects of designing and or identification of independently folding motifs from the existing protein structures. However, intriguingly, naturally occurring bab sequences from proteins that fold independently have not been identified. In our attempts we addressed the finding of ‘needles in hay stick’ scenario by an exhaustive sequence and structural space search of the bab units from the TIM barrels. Methodology & Theoretical Orientation: The search approach implemented in this work considered features such as alpha helical propensity, loop length, loop dynamics, residue preferences in loops, long range side chain main chain interactions etc., to shortlist bab units with strong propensity to fold in isolation. The prospective bab candidates thus shortlisted from the TIM barrels have been further subjected to structure forming tendency employing a combination of Monte Carlo and Molecular dynamics simulations to assess their foldability and stability. Conclusion & Significance: The prediction of some independently folding bab candidates from TIMs are enabling us to experimentally asses their folding and stability. The identification and analysis of independently folding bab units that exist naturally will not only provide substantial information on nature’s design strategies and evolution of protein conformations but also help to design/engineer novel proteins.
References:
- Grishin NV (2001). Fold change in evolution of protein structures. J Struct Biol. 134:167-85.
- Höcker B (2014). Design of proteins from smaller fragments-learning from evolution. Curr Opin Struct Biol. 27:56-62.
- Friedland GD, Kortemme T (2010). Designing ensembles in conformational and sequence space to characterize and engineer proteins. Curr Opin Struct Biol. 20:377-384.
- Riechmann L , Winter G (2006). Early protein evolution: building domains from ligand-binding polypeptide segments. J. Mol. Biol. 353:460-468
- Yang X, Kathuria SV, Vadrevu R, Matthews CR. (2009). Beta alpha- hairpin clamps brace beta alpha beta modules and can make substantive contributions to the stability of TIM barrel proteins. PLoS One. 4(9):e7179
- Special Session on Structural Biology of Biomembranes
Session Introduction
Qiu-Xing Jiang
University of Florida, USA
Title: Structural basis for the lipid-dependent gating of a Kv channel

Biography:
Qiu-Xing Jiang is currently heading the Laboratory of Molecular Biophysics and Cell Physiology in the Department of Microbiology and Cell Science in the Institute of Food and Agricultural Sciences of University of Florida (UF), and he is serving part-time (20%) as the faculty director of Electron Microscopy at the Institute of Cross-disciplinary Biotechnology Research (ICBR) at UF. His lab is working on lipid-dependent gating effects and on a newly discovered anion conductance in ithe regulated secretory pathways. Dr. Jiang obtained his PhD in 2002 from the Department of Cellular and Molecular Physiology at Yale University School of Medicine, where he started his work in cryo-electron microscopy (cryoEM) in 1999 with Dr. Fred Sigworth. He initiated the implementation of spherically constrained reconstruction (SCR) by imaging membrane proteins in small vesicles. After a short postdoctoral training at Yale, Dr. Jiang finished his postdoctoral training in structural biology with Dr. Roderick Mackinnon in 2007 before taking an Assistant Professorship position at UT Southwestern Medical Center at Dallas, Texas. During the time at Dallas, Dr. Jiang worked on the development of chemically functionalized carbon films for cryoEM imaging, and made new discoveries on the filament-based signal amplification in the innate immune response against RNA viruses. He studied membrane-induced pore formation by human C-type Lectin and the VopQ proteins, and discovered the mechanism for lipid-dependent gating of Kv channels. He has published 24 papers in international journals. He is the recipient of the NIH EUREKA award in 2009, the AHA National Innovative Award in 2012, and the Junior Faculty travel award from GRC Molecular and Cell Biology of Lipids in 2011.
Abstract:
Human cell membranes are made of both phospholipids and nonphospholipids. The nonphospholipids, such as cholesterol (CHOL), have no phosphate groups in their headgroup regions and are quite abundant in cell membranes. Mainly due to technical difficulties, quantitative study of possible effects of nonphospholipids on voltage-gated ion channels has been very challenging. Our prior studies have achieved three major developments: 1) a working hypothesis of lipid-dependent gating based on nonphospholipids stabilizing the voltage sensor domain (VSD) of the KvAP channel in the resting (“down”) conformation; 2) a novel bead-supported unilamellar membrane (bSUM) system and a new method to stabilize the KvAP channel in the resting state; and 3) chemically functionalized carbon (ChemiC) films for cryoEM imaging of low abundance complexes by high-affinity selection or of small macromolecular complexes (100-200 kDa) by keeping vitrified ice thinner than usual. The general idea for lipid dependent gating is that the annular lipids around a Kv channel change their arrangements in accompany with the conformational changes of the voltage-sensor domains. Our technical development made it feasible to study the CHOL-dependent gating effects on Kv channels. We studied the CHOL-dependent gating effects on Kv channels in bSUMs. Because almost all known lipid metabolic defects result from dysregulated homeostasis of nonphospholipids, our studies in animal models carrying CHOL metabolic defects will provide the first test of lipid-dependent gating in an in vivo physiological setting. Secondly, we apply our ChemiC method to cryoEM study of the 120 kDa KvAP in both an inactivated and a peptide-stabilized “down” state. The peptides selected from the nonphospholipid-stabilized down state have been showed to recognize the voltage sensors in the right conformation and keep the channels in the right conformation. Our results will reveal the structural basis for the nonphospholipid-induced conformational changes in Kv channels, and unveil connections to the lipid-metabolic defects in humans.
References:
- Hui Zheng, Weiran Liu, Lingyan Anderson, and Qiu-Xing Jiang. Lipid-dependent gating of a voltage-gated ion channel. Nature Comm., 2:250 doi:10.1038/ncomms1254, 2011.
- Marc Llaguno, Hui Xu, Liang Shi, Nian Huang, Hong Zhang, Qinghua Liu, and Qiu-Xing Jiang. (2014) Chemically functionalized nanometer-thick carbon films for single molecule imaging. J. Struct. Biol. 185(3):405-17.
- Hui Zheng, Sungsoo Lee, Marc Llaguno, and Qiu-Xing Jiang. (2016). bSUM: A bead-supported unilamellar membrane system facilitating unidirectional insertion of membrane proteins into giant vesicles. J Gen Physiol. 147(1):77-93.
- Hui Xu, Xiaojing He, Hui Zheng, Lily J. Huang, Fajian Hou, Zhiheng Yu, M. Jason de la Cruz, Brian Borkowski, Xuewu Zhang*, Zhijian J. Chen* and Qiu-Xing Jiang*. (2014) Structural basis for the prion-like MAVS filaments in antiviral innate immunity. eLife. 3:e01489. doi: 10.7554/eLife.01489.
- Sohini Mukherjee, Hui Zheng, Mehabaw Derebe, Keith Callenberg, Carrie L. Partch, Darcy Rollins, Daniel C. Propheter, Josep Rizo, Michael Grabe, Qiu-Xing Jiang*, and Lora V. Hooper*. (2014) Antibacterial membrane attach by a pore-forming C-type lectin. Nature, 505(7481): 103-7. (*co-senior authors).
John E. Baenziger
University of Ottawa, Canada
Title: Mechanisms underlying lipid-sensing by the nicotinic acetylcholine receptor in both normal and diseased states

Biography:
John Baenziger is a professor of Biochemistry at the University of Ottawa in Ottawa, Canada. His research is focused on understanding the mechanisms by which lipids influence nicotinic acetylcholine receptor structure and function in both normal and diseased states, with increasing focus on how lipid-nAChR interactions participate in congenital myasthenic syndromes. Dr. Baenziger has served on the editorial board of the Journal of Biological Chemistry. He is the President of the Biophysical Society of Canada and is Treasurer-elect of the International Union of Pure and Applied Biophysics.
Abstract:
The neuromuscular nicotinic acetylcholine receptor (nAChR) is the prototypic member of the pentameric ligand-gated ion channel (pLGIC) superfamily, a superfamily of neurotransmitter receptors that plays a central role in information processing in the brain. It is well documented that nAChR function is exquisitely sensitive to its lipid environment. Lipids influence function by both conformational selection and kinetic mechanisms – they stabilize different proportions of activatable versus non-activatable conformations, and influence the rates of transitions between the different states. In the absence of activing cholesterol and anionic lipids, the nAChR adopts a conformation where agonist binding is uncoupled from channel gating. Lipids likely influence the “coupling” of binding and gating via the lipid-exposed transmembrane a-helix, M4. M4 in the neuromuscular nAChR is also the site of both point and truncation mutations that alter expression and/or function leading to congenital myasthenic syndromes. In this seminar, I will focus on the mechanisms by which the peripheral M4 transmembrane a-helix modulates pLGIC function. The M4 C terminus extends beyond the bilayer to interact with key structures that link the agonist binding to the transmembrane gate – referred to here as the coupling interface. We hypothesized that interactions between M4 and the coupling interface are essential to pLGIC function. We show here that such interactions are essential to function in some pLGICs and do participate in lipid-sensing. In the neuromuscular nAChR, however, such interactions between M4 and the coupling interface are less important. Instead, M4 influences function via a cluster of polar residues located in the core of the transmembrane domain near the center of the lipid bilayer. Altered M4 structure leads to changes in the energetic coupling between these polar residues, with the changes coupling ultimately propagating to both the gating helix, M2, and the aforementioned coupling interface. Here, we map out the conformational pathway that leads from the lipid-exposed surface of M4 to the channel gate, and thus illustrate how M4 “allosterically” modulates channel function.
Figure 1: Some allosteric modulators, including lipids, act via the lipid-exposed M4 a-helix of the nAChR. We elucidate the allosteric pathway by which this peripheral structure influences channel gating.
References:
- Baenziger JE et al (2017) The role of cholesterol in the activation of nicotinic acetylcholine receptors. Current Topics in Membranes article in press
- Therien JPD, Baenziger JE (2017) Pentameric ligand-gated ion channels exhibit distinct transmembrane domain archetypes for folding/expression and function. Scientific Reports 7:450:1-14.
- Carswell CL et al (2015) Role of the fourth transmembrane a‑helix in the allosteric modulation of pentameric ligand-gated ion channels. Structure 23:1655-1664.
- Baenziger JE et al (2015) Nicotinic acetylcholine receptor-lipid interactions: mechanistic insight and biological function. BBA-Biomembranes 1848:1806-1817.
- daCosta CJB et al (2013) A novel mechanism for activating uncoupled nicotinic acetylcholine receptors. Nat Chem Biol 9:701-707
Biography:
Carsten Mim has a longstanding interest in membrane and membrane-associated proteins throughout his career. As an experienced electrophysiologist, he characterized the glutamate transporter EAAT3 and EAAT4. The kinetics of EAAT4 differ from other glutamate transporters, by a voltage sensitive step that slows the turnover rate at hyperpolarized membrane potentials. Further, recorded transient and steady state currents at different temperatures showed that the binding of glutamate is enthalpy-driven unlike the binding of Na+. To visualize membrane:protein complexes, he turned to electron microscopy. His work on the Bin/Amphyphysin/Rvs domain (BAR) protein endophilin in complex with the bilayer resulted in the unexpected discovery that the stability and dynamics of endophilin scaffolds entirely depend on non-specific interactions between amphipathic helices in the bilayer. His findings also provided a first structurally motivated hypothesis how BAR-scaffolds selectively recruit downstream interaction partners through a steric selection mechanism.
Abstract:
Statement of the Problem: The cell bends membranes to generate membrane structures, like the t-tubules in muscles. Bin1/Amphiphysin/Rvs domain (BAR) proteins are part of the membrane bending machinery and are found in widespread phenomena like endocytosis or cell motility. BAR domain proteins can assemble spontaneously in vitro as well as in vivo. Which factors regulate the assembly? The membrane tension is a well studied regulator. In contrast, the role of the membrane composition, as an initiator of membrane bending, is poorly understood. Methodology & Theoretical Orientation: For this study we collected electron micrographs to document the membrane bending activity of the BAR protein Bin1. We probed the electrostatic interactions between Bin1 and the membrane by changing the surface charge of the membrane, the ionic strength of the assay and using disease relevant mutants, where a positive charge (K35N) and a negative charge (D151N) are eliminated. The electrostatic interactions between Bin1 and artificial membranes were evaluated by liposome sedimentation. To test how the findings translate into living cells, we assayed the phenotype of membrane bending-deficient Bin1 mutants in cells that have elevated or reduced levels of negatively charged lipids. Findings: Our simple, artificial system could reproduce the complex membrane topology present in muscle cells. We focused on the two mutants. We found that in stringent conditions for membrane bending (high ionic strength, low membrane charge) the mutants showed disproportional lower bending activity. These finding were confirmed in vivo. We were able to rescue to mutant phenotype by increasing the membrane surface charge. Conversely, we induced a mutant phenotype in wt Bin1 by lowering the membrane surface charge. Conclusion & Significance: We established the membrane charge as a novel regulator of membrane tubulation. We speculate that rapid phosphorylation and dephosphorylation of phosphoinositols can act as a switch for induction of membrane bending.
References:
- Higher-order assemblies of BAR domain proteins for shaping membranes. Suetsugu S. Microscopy (Oxf). 2016 Feb 15
- Membrane tension and peripheral protein density mediate membrane shape transitions. Shi Z, Baumgart T., Nat Commun. 2015 Jan 8;6:5974
- Structural basis of membrane bending by the N-BAR protein endophilin. Mim C, Cui H, Gawronski-Salerno JA, Frost A, Lyman E, Voth GA, Unger VM., Cell 2012 Mar 30;149(1):137-45
- Protein-mediated transformation of lipid vesicles into tubular networks. Simunovic M, Mim C, Marlovits TC, Resch G, Unger VM, Voth GA. Biophys J. 2013 Aug 6;105(3):711-9

Biography:
Volodymyr Korkhov is an assistant professor at the Institute of Biochemistry and Paul Scherrer Institute (PSI, Villigen). Prof. Korkhov has been studying various aspects of membrane protein biology throughout his career. As a PhD student at the Institute of Pharmacology, Vienna Medical University, he studied oligomerization of neurotransmitter transporters. He continued research of multidrug and ABC transporters during his postdoctoral training periods at MRC Laboratory of Molecular Biology (Cambridge, UK) and ETH Zurich, respectively. His work on ABC transporter for vitamin B12 from Escherichia coli, BtuCDF, led to a proposal of a complete structure-based mechanism of type II ABC importers. From April 2014, Prof. Korkhov has been leading an independent research group, supported by an SNF Professorship. The overarching topic of research in Prof. Korkhov’s group is structure and molecular mechanisms of membrane protein complexes involved in signal transduction.
